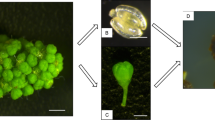
Abstract
Somatic embryogenesis (SE) offers vast potential for the clonal propagation of high-value roses. However, some recalcitrant cultivars unresponsive to commonly employed SE-inducing agents and low induction rates currently hinder the commercialization of SE technology in rose. Rose SE technology requires improvement before it can be implemented as a production system on a commercial scale. In the present work, we assessed 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), a synthetic auxin not previously tested in rose, for its effectiveness to induce SE in the rose cultivar ‘Livin’ Easy’ (Rosa sp.). We ran a parallel comparison to the commonly used 2,4-dichlorophenoxyacetic acid (2,4-D). We tested each auxin with two different basal media: Murashige and Skoog (MS) basal medium and woody plant medium (WPM). MS medium resulted in somatic embryo production, whereas WPM did not. 2,4,5-T induced SE over a greater concentration range than 2,4-D's and resulted in significantly greater embryo yields. 2,4,5-T at a concentration of 10 or 25 μM was better for embrygenic tissue initiation than 2,4,5-T at 5 μM. Further embryo development occurred when the tissue was transferred to plant growth regulator (PGR) free medium or media with 40% the original auxin concentration. However, the PGR-free medium resulted in a high percentage of abnormal embryos (32.31%) compared to the media containing auxins. Upon transfer to germination medium, somatic embryos successfully converted into plantlets at rates ranging from 33.3 to 95.2%, depending on treatment. Survival rates 3 months ex vitro averaged 14.0 and 55.6% for 2,4-D- and 2,4,5-T-derived plantlets, respectively. Recurrent SE was observed in 60.2% of the plantlets growing on germination medium. This study is the first report of SE in the commercially valuable rose cultivar ‘Livin’ Easy’ (Rosa sp.) and a suitable methodology was developed for SE of this rose cultivar.


Similar content being viewed by others
Abbreviations
- 2,4,5-T:
-
2,4,5-Trichlorophenoxyacetic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- MS medium:
-
Murashige and Skoog basal medium
- WPM medium:
-
Woody plant medium
- PGR:
-
Plant growth regulator
- ET:
-
Embryogenic tissue
- ABA:
-
Abscisic acid
- SAM:
-
Shoot apical meristem
- SE:
-
Somatic embryogenesis
References
Al-Mazrooei S, Bhatti MH, Henshaw GG, Taylor NJ, Blakeslay D (1997) Optimisation of somatic embryogenesis in fourteen cultivars of sweet potato [Ipomoea batatas (L.) Lam.]. Plant Cell Rep 16:710–714
Bangerth F, Li C-J, Gruber J (2000) Mutual interaction of auxins and cytokinins in regulatimg correlative dominance. Plant Growth Regul 32:205–217
Bowman JL, Eshed Y (2000) Formation and maintenance of the shoot apical meristem. Trends Plant Sci 5(3):110–115
Coca MA, Almoguera C, Jordano J. (1994) Expression of sunflower low-molecular-weight-heat-shock proteins during embryogenesis and persistence after germination: localization and possible functional implications. Plant Mol Biol 25:479–492
de Wit, JC, Esendam HF, Honhanen JJ, Tuominen U (1990) Somatic embryogenesis and regeneration of flowering plants in rose. Plant Cell Rep 9:456–458
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Eriksson T (1965) Studies on the growth requirements and growth measurements of cell cultures of Haplopapus gracilis. Physiol Plant 18:976–993
Fehér A, Pasternak T, Miskolczi P, Ayaydin F, Dudits D (2001) Induction of the embryogenic pathway in somatic plant cells. Acta Horticultur 560:293–298
Fehér A, Pasternak TP, Dudits D (2003). Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74(3):201–228
Hameed S, Ahmad Z, Khan FZ, Akram M (1993) Callus cultures of Rosa hybrida cvs. Diamond Jubly and Lans France. Pak J Bot 25(2):193–198
Hsia C, Korban SS (1996) Organogenesis and somatic embryogenesis in callus cultures of Rosa hybrida and Rosa chiensis minima. Plant Cell Tissue Organ Cult 44:1–6
Jeya Mary R, Jayabalan N (1997) Influence of growth regulators on somatic embryogenesis in sesame. Plant Cell Tissue Organ Cult 49:67–70
Kim SW, Seung CO, Dong SI, Liu JR (2003a) Plant regeneration of rose (Rosa hybrida) from embryogenic cell-derived protoplasts. Plant Cell Tissue Organ Cult 73:15–19
Kim CH, Chung J-D, Jee S-O, Oh J-Y (2003b) Somatic embryogenesis from in vitro grown leaf explants of Rosa hybrida L. J Plant Biotechnol 5(3):169–172
Kintzios S, Manos C, Makri O (1999) Somatic embryogenesis from mature leaves of rose (Rosa sp.). Plant Cell Rep 18:467–472
Kitamiya E, Suzuki S, Sano T, Nagata T (2000) Isolation of two genes that were induced upon the initiation of somatic embryogenesis on carrot hypocotyls by high concentrations of 2,4-D. Plant Cell Rep 19:551–557
Konar RN, Thomas E, Street HE (1972) Origin and structure of embryoids arising from eopidermal cells of the stem of Ranunculus sceleratus L. J Cell Sci 11:77–93
Kong L, Yeung EC (1992) Development of white spruce embryos II Continual shoot meristem development during germination. In vitro Cell Dev Biol 28:125–131
Kumria R, Sunnichan VG, Das DK, Gupta SK, Reddy VS, Bhatnagar RK, Leelavathi S (2003) High-frequency somatic embryo production and maturation into normal plants in cotton (Gossypium hirsutum) through metabolic stress. Plant Cell Rep 21(7):635–639
Kunitake H, Imamizo H, Mii M (1993) Somatic embryogenesis and plant regeneration from immature seed-derived calli of rugosa rose (Rosa rugosa Thunb.). Plant Sci 90:187–194
Lee EK, Cho DY, Soh WY (2001) Enhanced production and germination of somatic embryos by temporary starvation in tissue cultures of Daucus carota. Plant Cell Rep 20(5):408–415
Li X, Krasnyanski SF, Korban SS (2002) Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. J Plant Physiol 159:313–319
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb Proc Int Plant Propagators Soc 30:421–427
Marchant R, Davey MR, Lucas JA, Power BJ (1996) Somatic embryogenesis and plant regeneration in Floribunda rose (Rosa hybrida L.) cvs. Trumpeter and Glad Tidings. Plant Sci 120:95–105
Matthews D, Mottley J, Horan I, Roberts AV (1991) A protoplast to plant system in roses. Plant Cell Tissue Organ Cult 24:173–180
Matthews D, Mottley J, Yokoya K, Roberts AV (1994) Regeneration of plants from protoplasts of Rosa species (Roses). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 29. Springer-Verlag, Berlin, Heidelberg, pp 146–159
Merkle SA, Parrott WA, Williams EG (1990) Applications of somatic embryogenesis and embryo cloning. In: Bhojwani SS (ed) Plant tissue culture: applications and limitations. Elsevier Science Publishing Inc., Amsterdam, pp 67–101
Murali S, Sreedhar D, Lokeswari TS (1996) Regeneration through somatic embryogenesis from petal-derived calli of Rosa hybrida L. cv. Arizona (hybrid tea). Euphytica 91:271–275
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Noriega C, Söndahl MR (1991) Somatic embryogenesis in Hybrid Tea roses. Bio/Technol 9:991–993
Owen HR, Miller AR (1992) An examination and correction of plant tissue culture basal medium formulations. Plant Cell Tissue Organ Cult 28:147–150
Peres LEP, Kerbauy GB (1999) High cytokinin accumulation following root tip excision changes the endogenous auxin-to-cytokinin ratio during root-to-shoot conversion in Catasetum fimbriatum Lindl. (Orchidaceae). Plant Cell Rep 18:1002–1006
Reinert J (1958) Morphogenese und ihre Kontrolle an Gewebekulturen aus Karotten. Naturwissenschaften 45:344–345
Roberts AV, Horan I, Matthews D, Mottley J (1990) Protoplast technology and somatic embryogenesis in Rosa. In: de Jong J (ed) Integration of the in vitro techniques in ornamental plant breeding. CPO Centre for Plant Breeding Research, Wageningen, pp 100–115
Roberts AV, Yokoya K, Walker S, Mottley J (1995) Somatic embryogenesis in Rosa spp. In: Jain SM (ed) Somatic embryogenesis in woody plants. Kluwer Academic Publishers, Dordrecht, pp 277–289
Rout GR, Debata BK, Das P (1991) Somatic embryogenesis in callus cultures of Rosa hybrida L. cv. Landora. Plant Cell Tissue Organ Cult 27:65–69
Rout GR, Samantantaray S, Mottley J, Das P (1999) Biotechnology of the rose: a review of recent progress. Sci Horticultur 81:201–228
Sagare AP, Suhasini K, Krishnamurthy KV (1993) Plant regeneration via somatic embryogenesis in chickpea (Cicer arietinum L.). Plant Cell Rep 12:652–655
Sarasan V, Roberts AV, Rout GR (2001) Methyl laurate and 6-benzyladenine promote the germination of somatic embryos of a hybrid rose. Plant Cell Rep 20:183–186
Segura-Aguilar J, Hakman I, Rydstrom J (1995) Studies on the mode of action of the herbicidal effect of 2,4,5-trichlorophenoxyacetic acid on germinating Norway spruce. Environ Exp Bot 35(3):309–320
Skoog F, Miller CO (1965) Chemival regulation of growth and organ formation in plant tissues cultured in vitro. In: Bell E (ed) Molecular and cellular aspects of development. Harper and Row, New York, pp 481–494
Steward FC, Mapes MO, Mears K (1958) Growth and organized development of cultured cells: II. Organization of cultures grown from freely suspended cells. Am J Bot 45:705–708
Taiz L, Zeiger E (1998) Plant physiology, 2nd edn. Sinauer Associates Inc., Sutherland, Massachusetts, p 472
Van Der Salm TPM, Van Der Toorn CJG, Hänisch ten Cate CH, Dons HJM (1996) Somatic embryogenesis and shoot regeneration from excised adventitious roots of the rootstock Rosa hybrida L. ‘Moneyway’. Plant Cell Rep 15:522–526
Visessuwan R, Kawai T, Mii M (1997) Plant regeneration systems from leaf segment culture through embryogenic callus formation of Rosa hybrida and Rosa canina. Breeding Sci 47:217–222
Venkov P, Topashka-Ancheva M, Georgieva M, Alexieva V, Karanov E (2000) Genotoxic effect of substituted phenoxyacetic acids. Arch Toxicol 74(9):560–566
von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
von Arnold S, Bozhkov P, Clapham D, Dyachok J, Filonova L, Högberg, K-A, Ingouff M, Wiweger M (2005) Propagation of Norway spruce via somatic embryogenesis. Plant Cell Tissue Organ Cult 81(3):323–329
Witte CP, Tiller SA, Taylor MA, Davies HV (2002) Addition of nickel to Murashige and Skoog medium in plant tissue culture activates urease and may reduce metabolic stress. Plant Cell Tissue Organ Cult 68:103–104
Acknowledgements
We thank NSERC for the Postgraduate Industry Scholarship provided to T.E., Weeks Roses for supplying the rose material and PlantSelect Biotechnology Systems Ltd for both financial support and use of their facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zou
Rights and permissions
About this article
Cite this article
Estabrooks, T., Browne, R. & Dong, Z. 2,4,5-Trichlorophenoxyacetic acid promotes somatic embryogenesis in the rose cultivar ‘Livin’ Easy’ (Rosa sp.). Plant Cell Rep 26, 153–160 (2007). https://doi.org/10.1007/s00299-006-0231-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0231-5