Abstract
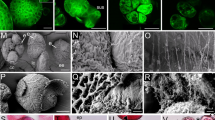
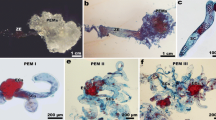
The Brassica napus secondary embryogenesis system requires no exogenous growth regulator to stimulate embryo development. It is stable embryogenically over a long period of culture and has a distinct pre-embryogenic stage. This system was used to investigate the morphological and cellular changes occurring in the embryogenic tissue compared to non-embryogenic tissue using various microscopy techniques. A unique ultrastructural feature designated the extracellular matrix (ECM) was observed on the surface of pre-embryogenic embryoids but not on the non-embryogenic individuals. The ECM layer was found to be dominant in the pre-embryogenic stage and reduced to fragments during embryo growth and development in mature embryogenic tissue. This is a novel aspect of the phenotype previously unreported in the Brassica system. This structure might be linked to acquisition of embryogenic competence.





Similar content being viewed by others
Abbreviations
- ECM :
-
Extracellular matrix
- EC :
-
Embryogenic
- PEC :
-
Pre-embryogenic
- NEC :
-
Non-embryogenic
- MEC :
-
Mature embryogenic
References
Bobak M, Blehova A, Kristin J, Ovecka M, Samaj J (1995) Direct plant regeneration from leaf explants of Drosera rotundifolia cultured in vitro. Plant Cell Tissue Organ Cult 43:43–49
Chapman A, Blervacq AS, Tissier JP, Delbreil B, Vasseur J, Hilbert JL (2000a) Cell wall differentiation during early somatic embryogenesis in plants. I. Scanning and transmission electron microscopy study on embryos originating from direct, indirect, and adventitious pathways. Can J Bot 78:816–823
Chapman A, Blervacq AS, Hendriks T, Slomianny C, Vasseur J, Hilbert JL (2000b) Cell wall differentiation during early somatic embryogenesis in plants. II. Ultrastructural study and pectin immunolocalisation on chicory embryos. Can J Bot 78:824–831
Chapman A, Blervacq AS, Vasseur J, Hilbert JL (2000c) Removal of the fibrillar network surrounding Cichorium somatic embryos using cytoskeleton inhibitors: analysis of proteic components. Plant Sci 150:103–114
Cook HC (1996) Carbohydrates. In: Bancroft JD, Stevens A (eds) Theory and practice of histological techniques. Churchill Livingstone, USA, pp 173–211
Dubois T, Guedira M, Dubois J, Vasseur J (1991) Direct somatic embryogenesis in leaves of Cichorium: a histological and SEM study of early stages. Protoplasma 162:120–127
Dubois T, Guedira M, Diop A, Vasseur J (1992) SEM characterization of an extracellular matrix around somatic proembryos of roots of Cichorium. Annals Bot 70:119–124
Jasik J, Salajova J, Salaj J (1995) Developmental anatomy and ultrastructure of early somatic embryos in European black pine (Pinus nigra Arn.). Protoplasma 185:205–211
Loh CS (1982) Towards the production of improved winter oilseed rape through tissue culture. PhD thesis, University of Cambridge, UK
Loh CS, Ingram DS (1982) Production of haploid plants from anther cultures and secondary embryoids of winter oilseed rape Brassica napus ssp. oleifera. New Phytol 91:507–516
Loh CS, Ingram DS (1983) The response of haploid secondary embryoids and secondary embryogenic tissues of winter oilseed rape to treatment with colchicines. New Phytol 95:359–366
Loh CS, Ingram DS, Hanke DE (1983) Cytokinins and the regeneration of plantlets from secondary embryoids of winter oilseed rape, Brassica napus ssp. oleifera. New Phytol 95: 349–358
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plantarum 15:473–497
Ovecka M, Bobak M, Blehova A, Kristin J (1998) Papaver somniferum regeneration by somatic embryogenesis and shoot organogenesis. Biol Plantarum 40:321–328
Pedroso MC, Pais MS (1992) A scanning electron microscopy and x-ray microanalysis study during induction of morphogenesis in Camellia japonica L. Plant Sci 87:99–108
Pedroso MC, Pais MS (1995) Factors controlling somatic embryogenesis. Cell wall changes as an in vivo marker of embryogenic competence. Plant Cell Tissue Org Cult 43:147–154
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208
Samaj J, Bobak M, Oecka M, Kristin J, Blehova A (1994) Extracellular matrix in early stages of plant regeneration in vitro. Cell Biol Int 18:545
Samaj J, Bobak M, Blehova A, Kristin J, Auxtova-Samajova O (1995) Developmental SEM observations on an extracellular matrix in embryogenic calli of Drosera rotundifolia and Zea mays. Protoplasma 186:45–49
Samaj J, Ensikat H-J, Baluska F, Knox JP, Barthlott W, Volkmann D (1999a) Immunogold localization of plant surface arabinogalactan-proteins using glycerol liquid substitution and scanning electron microscopy. J Microscopy 193:150–157
Samaj J, Baluska F, Bobak M, Volkmann D (1999b) Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep 18:369–374
Shu W, Loh CS (1987) Secondary embryogenesis in long term cultures of winter oilseed rape, rape Brassica napus ssp. oleifera. New Phytol 107:39–46
Sondahl MR, Salisbury JL, Sharp WR (1979) SEM characterization of embryogenic tissue and globular embryos during high frequency somatic embryogenesis in coffee callus cells. Zeitschrift für Pflanzenphysiologie l 94:185–188
Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferriere N, Nicole M (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot 88:9–18
Verdus MC, Dubois T, Dubois J, Vasseur J (1993) Ultrastructural changes in leaves of Cichorium during somatic embryogenesis. Ann Bot 72:375–383
Acknowledgements
We are grateful to Ms. Janet Powell and Mr. Tony Burgess (Multi Imaging Centre, Department of Anatomy, University of Cambridge, UK) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. E. Horn
Rights and permissions
About this article
Cite this article
Namasivayam, P., Skepper, J. & Hanke, D. Identification of a potential structural marker for embryogenic competency in the Brassica napus spp. oleifera embryogenic tissue. Plant Cell Rep 25, 887–895 (2006). https://doi.org/10.1007/s00299-006-0122-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0122-9