Summary
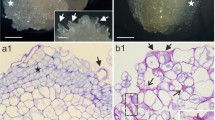
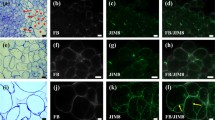
Primary embryogenic callus ofDrosera rotundifolia and long-term cultured embryogenic callus ofZea mays possess a conspicuous extracellular matrix (ECM) around and between embryogenic cells. The structural arrangement of ECM depends on the developmental stage of the embryogenic cells. Single embryoid cells were covered with, and connected by net-like material. However, surface cells of young globular embryoids were covered with a coherent layer of ECM which forms bridges with net-like material between the cells which was gradually reduced to coarse strands. When protodermis was formed on the surface of globular embryoids, the ECM disappeared completely. The ECM network was never observed on the surface of heart- and torpedo-shaped embryoids. Safranine (especially 0.1%) stabilized the structure of ECM. Digestion with pronase E and proteinase K indicated that the ECM contains proteinaceous components. Similar developmental patterns of ECM were observed in dicotyledonous and monocotyledonous examples. The ECM represents a stable morphological structure even during long-term embryogenic culture in maize.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- Dicamba:
-
3,6-dichloro-o-anisic acid
- ECM:
-
extracellular matrix
- KIN:
-
kinetin
- SEM:
-
scanning electron microscopy
- TEM:
-
transmission electron microscopy
References
Arborgh B, Bell P, Brunk U, Collins VP (1976) The osmotic effects of glutaraldehyde during fixation. A transmission electron microscopy, scanning electron microscopy and cytochemical study. J Ultrastruct Res 56: 339–350
Ausprunk DH (1981) Proteoglycans in the microvasculature. Am J Pathol 103: 353–375
Chen K, Wight TN (1984) Proteoglycans in arterial smooth muscle cell culture. J Histochem Cytochem 32: 347–357
Cohen AL (1974) Critical point drying. In: Hayat MA (ed) Principles and techniques of scanning electron microscopy. Van Nostrand Reinhold, New York, pp 44–112
Dubois T, Guedira M, Dubois J, Vasseur J (1991) Direct somatic embryogenesis in leaves ofCichorium. A histological and SEM study of early stages. Protoplasma 162: 120–127
—, Dubois J, Guedira M, Diop A, Vasseur J (1992) SEM characterization of an extracellular matrix around somatic proembryos in roots ofCichorium. Ann Bot 70: 119–124
van Engelen FA, de Vries SC (1992) Extracellular proteins in plant embryogenesis. Trends Genet 8: 66–70
Jásik J, Salajová T, Salaj J (1995) Developmental anatomy and ultrastructure of early somatic embryos in european black pine (Pinus nigra Arn.). Protoplasma 185: 205–211
Kachar B, Parakkal M, Frex J (1990) Structural basis for mechanical transduction in the frog vestibular sensory apparatus: I. The otolithic membrane. Hearing Res 45: 179–190
McCann MC, Wells B, Roberts K (1990) Direct visualization of cross-links in the primary plant cell wall. J Cell Sci 96: 323–334
Schiff RI, Gennaro JF (1979) The role of the buffer in the fixation of biological specimens for the transmission and scanning electron microscopy. Scanning 2: 135–148
Sondahl MR, Salisbury JL, Sharp WR (1979) SEM characterization of embryogenic tissue and globular embryos during high-frequency somatic embryogenesis in coffee callus cells. Z Pflanzenphysiol 94: 185–188
Sterk P, Booij H, Schellekens GA, van Kammen A, de Vries SC (1991) Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell 3: 907–921
de Vries SC, Booij H, Janssens R, Vogels R, Saris L, Loschiavo F, Terzi M, van Kammen A (1988) Carrot somatic embryogenesis depends on the phytohormone-controlled presence of correctly glycosylated extracellular proteins. Genes Dev 2: 462–476
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Šamaj, J., Bobák, M., Blehová, A. et al. Developmental SEM observations on an extracellular matrix in embryogenic calli ofDrosera rotundifolia andZea mays . Protoplasma 186, 45–49 (1995). https://doi.org/10.1007/BF01276934
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276934