Abstract
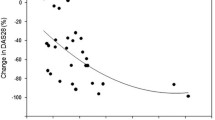
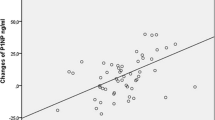
Juvenile idiopathic arthritis (JIA) is an inflammatory disease associated with bone loss and low bone mineral density (BMD). The treatment involves disease-modifying antirheumatic drugs, glucocorticoids (GCs) and biological agents. The aim of this study was to evaluate effects of 12-month therapy with the anti-tumor necrosis factor alpha (anti-TNFα) preparations on bone mineral density (BMD) and biochemical turnover markers (BTM) in adult patients with JIA who were previously either treated or not treated with glucocorticoids (GC) and to assess effects of the discontinuation of GCs on their bone status. Nineteen adult patients (12 women, 7 men) aged 18–33 years with active JIA were prospectively enrolled to receive the anti-TNFα therapy (infliximab, etanercept or adalimumab). BMD and BTMs were determined at baseline and 1-year follow-up. The anti-TNFα therapy resulted in a significant reduction in disease activity score 28 (DAS28) and C-reactive protein (CRP) and a significant increase in BMD at the lumbar spine and total body and in serum N-terminal propeptide of type I procollagen (PINP, marker of bone formation). No significant changes in serum beta C-terminal telopeptide of type I collagen (βCTX, marker of osteoclastic bone resorption) and osteocalcin (marker of bone remodeling) were found. A significant negative correlation was observed between the change in the DAS28, CRP and serum PINP. The change in serum PINP concentrations positively correlated with the change in lumbar spine BMD. A significant increase in serum PINP was observed only in patients discontinuing GCs during the anti-TNFα treatment. After the initiation of the anti-TNFα therapy in young adults with JIA, the increase in new bone formation can be explained by discontinuation of GCs administration as the patients with the largest reduction in DAS28 and CRP probably are the ones most likely to stop GC.



Similar content being viewed by others
References
Pepmueller PH, Cassidy JT, Allen SH, Hillman LS (1996) Bone mineralization and bone mineral metabolism in children with juvenile rheumatoid arthritis. Arthr Rheum 39:746–757
Henderson CJ, Specker BL, Sierra RI, Campaigne BN, Lovell DJ (2000) Total-body bone mineral content in non-corticosteroid-treated postpubertal females with juvenile rheumatoid arthritis: frequency of osteopenia and contributing factors. Arthr Rheum 43:531–540
Reeve J, Loftus J, Hesp R, Ansell BM, Wright DJ, Woo PM (1993) Biochemical prediction of changes in spinal bone mass in juvenile chronic (or rheumatoid) arthritis treated with glucocorticoids. J Rheumatol 20:1189–1195
Lien G, Flato B, Haugen M, Vinje O, Sorskaar D, Dale K, Johnston V, Egeland T, Forre O (2003) Frequency of osteopenia in adolescents with early-onset juvenile idiopathic arthritis: a long-term outcome study of one hundred five patients. Arthr Rheum 48:2214–2223
Zak M, Hassager C, Lovell DJ, Nielsen S, Henderson CJ, Pedersen FK (1999) Assessment of bone mineral density in adults with a history of juvenile chronic arthritis: a cross-sectional long-term followup study. Arthr Rheum 42:790–798
Burnham JM, Shults J, Weinstein R, Lewis JD, Leonard MB (2006) Childhood onset arthritis is associated with an increased risk of fracture: a population based study using the general practice research database. Ann Rheum Dis 65:1074–1079
Brabnikova Maresova K (2011) Secondary osteoporosis in patients with juvenile idiopathic arthritis. J Osteoporos. doi:10.4061/2011/569417
Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H et al (1994) Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 344:1105–1110
Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC, Furst DE, Mease PJ, Ruderman EM, Horwitz DA, Arkfeld DG, Garrison L, Burge DJ, Blosch CM, Lange ML, McDonnell ND, Weinblatt ME (1999) Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 130:478–486
Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore J, Finck BK (2000) Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric rheumatology collaborative study group. N Engl J Med 342:763–769
Lovell DJ, Giannini EH, Reiff A, Jones OY, Schneider R, Olson JC, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Lange M, Finck BK, Burge DJ (2003) Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthr Rheum 48:218–226
Quartier P, Taupin P, Bourdeaut F, Lemelle I, Pillet P, Bost M, Sibilia J, Kone-Paut I, Gandon-Laloum S, LeBideau M, Bader-Meunier B, Mouy R, Debre M, Landais P, Prieur AM (2003) Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthr Rheum 48:1093–1101
Lahdenne P, Vahasalo P, Honkanen V (2003) Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis 62:245–247
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalised bone loss in patients with early rheumatoid arthritis. Lancet 344:23–27
Vojvodich PF, Hansen JB, Andersson U, Savendahl L, Hagelberg S (2007) Etanercept treatment improves longitudinal growth in prepubertal children with juvenile idiopathic arthritis. J Rheumatol 34:2481–2485
Tynjala P, Lahdenne P, Vahasalo P, Kautiainen H, Honkanen V (2006) Impact of anti-TNF treatment on growth in severe juvenile idiopathic arthritis. Ann Rheum Dis 65:1044–1049
Simonini G, Giani T, Stagi S, de Martino M, Falcini F (2005) Bone status over 1 year of etanercept treatment in juvenile idiopathic arthritis. Rheumatology (Oxford) 44:777–780
Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 26:1729–1739
Simon D, Fernando C, Czernichow P, Prieur AM (2002) Linear growth and final height in patients with systemic juvenile idiopathic arthritis treated with longterm glucocorticoids. J Rheumatol 29:1296–1300
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Baim S, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Lewiecki EM, Silverman S (2008) Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom 11:75–91
Lovell DJ, Reiff A, Jones OY, Schneider R, Nocton J, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore JB, White B, Giannini EH (2006) Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthr Rheum 54:1987–1994
Goldring SR (2003) Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology (Oxford) 42(Suppl 2):ii11–16
Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast-Akrad M, Lang S, Turk B, Pietschmann P, Woloszczuk W, Haralambous S, Kollias G, Steiner G, Smolen JS, Schett G (2002) Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthr Rheum 46:785–792
Viswanathan A, Sylvester FA (2008) Chronic pediatric inflammatory diseases: effects on bone. Rev Endocr Metab Disord 9:107–122
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL (2005) IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 115:282–290
Barnabe C, Hanley DA (2009) Effect of tumor necrosis factor alpha inhibition on bone density and turnover markers in patients with rheumatoid arthritis and spondyloarthropathy. Semin Arthritis Rheum 39:116–122
Strand V, Kavanaugh AF (2004) The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology (Oxford) 43(Suppl 3):iii10–iii16
Michalska D, Stepan JJ, Basson BR, Pavo I (2006) The effect of raloxifene after discontinuation of long-term alendronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 91:870–877
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Vanden Berghe W, Vermeulen L, Delerive P, De Bosscher K, Staels B, Haegeman G (2003) A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv Exp Med Biol 544:181–196
Nanes MS (2003) Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene 321:1–15
Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y (2004) IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 15:49–60
Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN (2007) Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NFkappaB. J Bone Miner Res 22:646–655
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163
Billiau AD, Loop M, Le PQ, Berthet F, Philippet P, Kasran A, Wouters CH (2010) Etanercept improves linear growth and bone mass acquisition in MTX-resistant polyarticular-course juvenile idiopathic arthritis. Rheumatology (Oxford) 49:1550–1558
Malik S, Wong SC, Bishop J, Hassan K, McGrogan P, Ahmed SF, Russell RK (2011) Improvement in growth of children with Crohn disease following anti-TNF-alpha therapy can be independent of pubertal progress and glucocorticoid reduction. J Pediatr Gastroenterol Nutr 52:31–37
Rahimi R, Nikfar S, Abdollahi M (2007) Meta-analysis technique confirms the effectiveness of anti-TNF-alpha in the management of active ulcerative colitis when administered in combination with corticosteroids. Med Sci Monit 13:PI13–PI18
Vis M, Havaardsholm EA, Haugeberg G, Uhlig T, Voskuyl AE, van de Stadt RJ, Dijkmans BA, Woolf AD, Kvien TK, Lems WF (2006) Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 65:1495–1499
Thayu M, Leonard MB, Hyams JS, Crandall WV, Kugathasan S, Otley AR, Olson A, Johanns J, Marano CW, Heuschkel RB, Veereman-Wauters G, Griffiths AM, Baldassano RN (2008) Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: results of the REACH study. Clin Gastroenterol Hepatol 6:1378–1384
Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J (2000) The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 11 Suppl 6:S2–17
Hofbauer LC, Rauner M (2009) Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol Endocrinol 23:1525–1531
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282
Szappanos A, Toke J, Lippai D, Patocs A, Igaz P, Szucs N, Futo L, Glaz E, Racz K, Toth M (2010) Bone turnover in patients with endogenous Cushing’s syndrome before and after successful treatment. Osteoporos Int 21:637–645
Acknowledgments
We would like to acknowledge the professional cooperation by Mrs. Ludmila Hauptvoglova, Blanka Runstukova, Vladislava Trojanova, Pavlina Bobrova, Katerina Vondrickova, Jirina Friedova, Marie Cerna, Slavka Ferdanova and Hana Janochova. The study fully supported by the Grant Agency of Charles University in Prague, GAUK 84208/2008 and by the Ministry of Health Research Grant MZd CR 000 2372.
Conflict of interest
The authors stated that there is no conflict of interest regarding the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brabnikova Maresova, K., Jarosova, K., Pavelka, K. et al. Bone status in adults with early-onset juvenile idiopathic arthritis following 1-year anti-TNFα therapy and discontinuation of glucocorticoids. Rheumatol Int 33, 2001–2007 (2013). https://doi.org/10.1007/s00296-013-2678-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-013-2678-3