Abstract
The fabrication of crosslinked polyaniline/Pterocladia capillacea-activated carbon composite (CrossPANI/P-AC) at different ratios (1:0, 1:0.2, 1:0.6, and 1:1) was studied. CrossPANI/P-AC composites were fabricated by the in situ polymerization of aniline using hydrogen chloride as an acidic dopant, and ammonium persulfate as initiator, while Pterocladia capillacea-activated carbon was synthesized by the chemical activation method and incorporated into the polymer matrix. The samples were characterized by the terms such as Fourier transform infrared (FTIR) spectroscopy, Brunauer–Emmett–Teller, X-ray diffraction (XRD), thermogravimetric analysis, scanning electron microscopy, and energy-dispersive X-ray spectroscopy (EDX). FTIR spectroscopy showed the main characteristic peak positions of CrossPANI/P-AC; XRD showed low crystallinity of CrossPANI/P-AC. A high specific surface area for CrossPANI/P-AC was achieved at a ratio of 1:0.2 where Brunauer–Emmett–Teller surface area, total pore volume, and mean pore diameter values were 166.10 m2/g, 0.0141 cm3/g, and 3.40 nm, respectively. The capability of CrossPANI/P-AC (1:0.2) composite as adsorbent for Direct blue-86 (DB-86) dye from aqueous solution was investigated. The impact of initial dye concentration, temperature, pH, and contact time on the DB-86 dye adsorption from its water solution was examined. The equilibrium adsorption data were well represented by the Langmuir isotherm achieving maximum monolayer capacity (Qm) of 163.93 mg/g at a dose of 0.5 g/L. In contrast, the kinetic adsorption data were well fit by the pseudo-second-order model. Thermodynamic analysis demonstrated that DB-86 dye adsorption occurs spontaneously, endothermically, and physically in nature. The results demonstrated that these composites effectively removed DB-86 dye from aqueous solutions and could be recycled.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is the most valuable human resource on the planet [1], and it necessitates continuous examination and adjustment of water resource policy at all levels. Water pollution has been identified as the most prominent global cause of mortality and disease [2,3,4], with water contamination responsible for the deaths of 1.8 million people in 2015 [5]. The introduction of numerous colored chemicals into diverse water bodies due to increasing industrialization and urbanization has recently caused a major imbalance in aquatic systems [6]. According to the number of workers, export prices, and local production costs, Egypt's textile industry is one of the country's most important [7]. The textile industry sector, in particular, produces a lot of dye-bearing effluents [8]. Anionic dyes, cationic dyes, and non-ionic dyes are the three types of dyes, and the majority of them are harmful, and some are cancerous [8, 9]. Direct Blue 86 dye (Color Index No. 74180, CAS No. 1330–38-7), also called Direct Fast Blue GL or Direct Fast Turquoise Blue GL, having chemical formula C32H14CuN8Na2O6S2, is a gray-blue to blue powder, well soluble in water; the solution is lake blue [10]. It is an anionic dye used in the dyeing of cellulosic fabrics such as cotton, flax, viscose rayon, acetate, and jute. It can also be used to color leather and paper [11].
A number of physical or chemical processes were applied for dye treatment [12]. Chemical coagulation [13], chemical oxidation [14], ozonation [7, 15,16,17,18,19,20], irradiation [21,22,23,24,25,26], electrochemical oxidation [27], biological process [28], precipitation [29], and adsorption [30,31,32,33,34] are some of these methods. Because of its accuracy, ease of operation, lack of sensitivity to harmful compounds, and capacity to treat concentrated colored solutions, adsorption is a practical and cost-effective approach for removing colors [35]. Many adsorbing materials are used for the adsorption process, including activated alumina, silica gel, activated carbon [30,31,32,33,34,35,36], molecular sieve carbon, molecular sieve zeolites, polymeric [37], and polymer composite [38,39,40].
Recently, carbon-based/polymer composites have been investigated for water treatment, where it easily obtained and characterized. Polymer/activated carbon composite is considered a low-cost adsorbent for removing different pollutants from aquatic environments [39, 40]. The research focused on producing effective activated carbons alternatives to commercial activated carbon by employing biomaterials to make cheap and effective activated carbon [30,31,32,33,34,35,36]. There are several suitable biomaterials used to prepare activated carbon, including Ulva lactuca [34], orange peel [36], Pisum sativum peels [30], Pea peels [31], Pterocladia capillacea [41]. Marine biomaterial Pterocladia capillacea is available in the Mediterranean, and its chemically activated carbon by zinc chloride achieved a highly specific surface area under the optimized conditions at activation temperature 600 °C, holding time 30 min, and 1 M HCl soaked was 1202.70 m2/g [41].
Polyaniline (PANI), a conducting polymer with unusual characteristics, is one of the oldest researched polymers [42, 43]. Because of its low cost, ease of synthesis, good thermal and electrical properties, and environmental stability, PANI is a very promising polymer [44, 45]. As a result, it is widely employed in various disciplines, including rechargeable batteries, sensors, electrode materials, electro-catalysis, and anticorrosion [45]. The imine-to-amine nitrogen ratio in polyaniline can exist in three different oxidant states [46, 47]. Specifically, emeraldine base (EB) is a half-reduced form, leucoemeraldine (LB) is a fully reduced form, and pernigrani is a fully oxidized form [48]. PANI was synthesized by two kinds of polymerization: one is the chemical oxidation and the other is the electrochemical method [45]. Recently, polyaniline (PANI), CrossPolyaniline (CrossPANI) and their composites have recently demonstrated very high removal capacity for heavy metals such as Cr(VI) [49,50,51,52,53,54,55,56], Cr(III) [57], Zn(II) [58], Cd(II) [59], Pb(II) [60], Cu(II) [61], Hg(II) [61], dyes such as Direct Green 6 [62], Reactive Orange 16 [62], methyl orange [63], methylene blue [63], Reactive red 198 [64], Brilliant Green [65].
Therefore, the aim of this work was to fabricate crosslinked polyaniline/Pterocladia capillacea-activated carbon composites (CrossPANI/P-AC) as adsorbents and study their characterization using X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), and Brunner–Emmett–Teller (BET) surface area analysis. Furthermore, we estimate the adsorption behavior of CrossPANI/P-AC for removing of the Direct Blue 86 dye (an anionic dye) from the aquatic environment.
Materials and methods
Chemicals
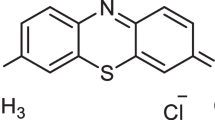
Aniline (C6H5NH2, M.W = 93.13 g, Assy = 99.5%) was purchased from Loba Chemie PVT. LTD, India. Ammonium persulfate [(NH4)2S2O8, M.W = 228.19 g, Assy = 98%] was purchased from Oxford Lab Chem (Navghar Road, India). Hydrochloric acid (HCl, M.W = 36.46 g, Assy = 30–34%) was obtained from SD Fine-Chem Limited, Mumbai, India. Sodium hydroxide (NaOH, M.W = 40 g, Min. Assy 96%) and ethanol (C2H6O, M.W = 46.07 g, Assy = 99.5%) were purchased from ADWIC, El Nasr pharmaceutical chemical company, Egypt. Sulfuric acid (H2SO4, M.W = 98.07 g, Assy = 98.0%) purchased from SD Fine-Chem Limited, Mumbai, India. Direct Blue–86, Chemical formula C32H14O6N8S2CuNa2 (M.W = 780.2 g) obtained from Sigma-Aldrich, USA (Fig. 1). All of the substances used in this experiment were analytical-grade reagents. All of the solutions and reagents were made with distilled water.
Synthesis of Pterocladia capillacea-activated carbon (PC-AC) nanoparticles
Pterocladia capillacea-activated carbon (PC-AC) nanoparticles were prepared by the chemical activation method [41]. In briefly, Pterocladia capillacea was collected from the Mediterranean, washed with distilled water, dried in an oven then milled, crushed and stored until used. The dried Pterocladia capillacea (10 g) was soaked with solution of ZnCl2 (5 g) for 24 h. After that the mixtures were dehydrated in 24 h at 110 °C and then pyrolysis in a quartz tube under N2 atmosphere at carbonized temperature 600 °C, holding time 30 min. The activated carbon was chilled at ambient temperature before being washed with boiling water and dried in a 70 °C oven. The samples were then refluxed in a Soxhlet extractor for 2 h with 1 M HCl, then filtered and washed with distilled water until pH was neutral. The activated carbon was then dried at 70 °C, crushed, sieved smaller than 100 mm, and stored in a glass bottle until needed.
Synthesis crosslinked Polyaniline/Pterocladia capillacea-activated carbon composite (CrossPANI/P-AC)
Polyaniline (PANI) was synthesized on the surface of the P-AC with ratio (1:0, 1:0.2, 1:0.6, 1:1) as follows: aniline and Pterocladia capillacea-activated carbon were premixed in HCl (1 M) for 2 h under cooling the mixture below 5 °C using ice bath. A pre-cooled (NH4)2S2O8 (0.3 M) in HCl solution (1 M) was slowly added under stirring to the monomer (aniline) solution for 30 min. The reaction vessel was placed in an ice bath cooling system during oxidation cooling since the reaction is highly exothermic (ΔH = 372 kJ mol−1). After complete oxidation, the reaction mixture was stirred for two hours at low temperature (0–5 °C) and then left unstirred overnight at room temperature. The precipitated polymer (dark blue powder) was filtered, washed with copious amounts of distilled water, and then diluted with HCl solution until the washing liquid was colorless. Then, we washed with ethanol to remove oligomers and non-polymeric impurities and dried under vacuum at 50 °C to produce the aimed material. Heating PANI/P-AC produced CrossPANI/P-AC at 180 °C for 3 h [61].
Characterization
X-ray diffraction (XRD) patterns were obtained using Bruker's X-ray diffractometer (model: 2D Phaser, Germany), in the range 2θ: 10°–80°, with CuKα radiation (λ = 1.540598 Ǻ). FTIR was studied using Bruker VERTEX70 Spectrometer connected with platinum ATR (model V-100, Germany) over the range of (400—4000 cm–1) to determine the chemical bonds and functional groups. Thermal stabilities studies were operated by Simultaneous Thermal Analyzer (model: SDT650, USA) instrument in the temperature range of room temperature to 900 °C, with a ramping temperature of 5 °C per minute under an atmosphere of nitrogen gas (100 mL/min). The Brunner–Emmett–Teller (BET) surface areas were determined by N2 adsorption–desorption isotherm using (BELSORP – model: Mini II, BEL Japan). The morphological structure was performed using a scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopy (EDX) using (JEOL-JSM-5300 LV, Tokyo, Japan). The point of zero charges (pHpzc) was determined as described in [66] using pH meter JENCO (model 6173, USA). UV–visible spectra of direct blue 86 dye before and after adsorption were recorded on PG instrument (model T80, UK) spectrophotometer.
Removal of Direct Blue 86 dye by batch method
Direct Blue 86 dye stock solution (1000 mg/L) was prepared by dissolving 1.0 g of DB-86 dye in 1000 ml distilled water. All working solutions with various concentrations were obtained by successive dilution with distilled water. Measurement of DB-86 dye concentration was carried out by spectrophotometer at (λmax = 615 nm) using a standard curve. The removal of DB-86 dye was performed via batch equilibrium method. The removal % of DB-86 dye using CrossPANI/P-AC composites with different ratios was determined. The influence of pH, contact time, CrossPANI/P-AC dosage, initial DB-86 dye concentration and adsorption temperature were investigated.
The removal % of DB-86 dye on CrossPANI/P-AC with different ratios (1:0, 1:0.2, 1:0.6, 1:1) was tested by adding 50 mg of each composite to 100 mL of 50 mg/L DB-86 dye solution at pH 2.2, and shacking at 200 rpm for 180 min contact time. The adsorption capacity of CrossPANI/P-AC for DB-86 dye is calculated from equation:
where qe is the adsorption capacity of DB-86 dye-loaded adsorbent (mg/g), C0 and Ce represent the initial and equilibrium concentrations of DB-86 dye (mg/L), m is the amount of CrossPANI/P-AC (mg), and V is the volume (L) of DB-86 dye solution.
The effect of solution pH on the adsorption was conducted by adding 0.05 g of CrossPANI/P-AC (1:0.2) composite to 100 mL of 50 mg/L DB-86 dye solution at pH range from 2.2 to 8.5 adjusted using JENCO pH meter and shaken in a shaker operated at 200 rpm at 27 ± 1 °C. After three hours, an appropriate amount of the reaction solution was taken out and then centrifuged, and the remained concentration were measured.
The effect of adsorbent mass on DB-86 dye adsorption was investigated by adding different amounts of CrossPANI/P-AC (1:0.2) (0.05, 0.075, 0.1, 0.125, and 0.150 g) into a number of flasks containing 100 mL known DB-86 dye solutions concentration and shaking at 200 rpm at 27 ± 1 °C to the equilibrium uptake (180 min) by determining the amount of DB-86 dye adsorption was.
At pH 2.2, shaking speed 200 rpm, and temperature 27 ± 1 °C, the effect of time and kinetics experiments was carried out in a series of flasks containing 0.05 g of adsorbent and 100 mL of DB-86 dye of various concentrations (50–150 mg/L) at time intervals of 5–180 min. At predetermined intervals, an appropriate amount of the solution was taken out and then centrifuged, and concentration was determined. The linear kinetic models such as pseudo-first-order, pseudo-second-order, Elovich, intraparticle diffusion, and film diffusion were used to generate the theoretical kinetics graphic (Table 1) [67,68,69,70,71].
The effect of initial concentrations was investigated at temperature 25 ± 1 °C and pH 2.2 by shaking (0.05, 0.075, 0.1, 0.125, 0.15) g of adsorbent CrossPANI/P-AC(5:1) with 100 mL of DB- 86 solution of various concentrations from (50, 75, 100, 125, 150 mg/L) and shaken in a shaker operated at 200 rpm. The adsorbent was removed by centrifugation after equilibrium, the supernatant was collected, and the concentration of DB-86 dye that remained was measured. Table 2 summarizes the adsorption isotherms for removing DB-86 dye using the Langmuir, Freundlich, Tempkin, and Dubinin–Radushkevich isotherm equations. Coefficient of determination (R2) and average percentage errors (APE%) function determined using Eq. (6) were used to determine the validity of the isotherm models fitted by the nonlinear regression method [68].
The temperature effect on the adsorption process of DB-86 dye was investigated by adding 0.05 g of CrossPANI/P-AC (1:0.2) into flasks containing 100 mL solutions of 50 mg/L dye concentration at temperatures 35, 40, 45, and 50 ± 1 °C. The parameters of thermodynamic study such as Gibbs free energy (ΔG, J/mol), entropy change (ΔS, J/K mol) and enthalpy change (ΔH, J/mol) use van’t Hoff Eqs. (11, 12, 13) [78]:
where Kd is distribution coefficient (Kd = qe/Ce, L/g), R (8.314 J/mol K), T (K) are the universal gas constant and the absolute temperature, respectively. The values of ΔH and ΔS were determined from the plot of ln Kd versus 1/T.
Desorption experiments were studied through the following two steps. First, the samples utilized in the adsorption experiments performed with 50 mg/L initial concentration of DB-86 dye solution and 50 mg CrossPANI/P-AC(1:0.2) dose at pH 2.2 conditions and shaking at speed 200 rpm for three hours were collected and then dried at 70 °C. Secondly, 50 mg of DB-86 dye-loaded composites was immersed in 100 mL elution media NaOH (0.1 M) under shaking at a speed of 200 rpm at a temperature 27 ± 1 °C for 2 h, and then, the sample was rinsed with distilled water several times until neutral pH and dried. The eluted DB-86 dye per gram of adsorbent (qdes) from the concentration of DB-86 dye desorbed (Cdes) in the solution is calculated by Eq. (14).
where V is the volume of the eluent used in liters and m is the weight of the adsorbent in grams. Desorption % was calculated by comparing DB-86 dye desorbed (qdes) to the DB-86 dye adsorbed (q) using Eq. (15).
Three adsorption–desorption cycles were performed on the samples to ensure that the composites could function as practical DB-86 dye adsorbents.
Results and discussion
Characterization
SEM and EDX analysis
The surface morphology of CrossPANI/P-AC (1:0), Cross PANI/P-AC(1:0.2), and DB-86 dye adsorbed onto CrossPANI/P-AC (1:0.2) was obtained by SEM and is presented in Fig. 2a, b, c, respectively. CrossPANI/P-AC (1:0) shows its relatively amorphous structure, and CrossPANI/P-AC (1:0.2) indicates that the polymerization has occurred on the surface of P-AC. After adsorption of DB-86 dye, the surface became more a denser structure, indicating that adsorption occurred on the surface of the CrossPANI/P-AC (1:0.2).
EDX data of CrossPANI/P-AC (1:0) and CrossPANI/P-AC (1:0.2) showed that the overall amount of the carbon (C), nitrogen (N), and oxygen (O) elements present in the fabricated composite were estimated using EDX analysis that confirmed the presence of PANI[(C6H5NH-)n] (Fig. 3). Besides, sulfur and chloride were also presented in the EDX analysis, presumably due to ammonium persulfate (APS) and the reaction medium, respectively. The mass % of carbon on CrossPANI/P-AC (1:0.2) is higher than in CrossPANI/P-AC (1:0) due to addition of P-AC.
FTIR analysis
Figure 4 shows FTIR spectra of P-AC, CrossPANI/P-AC (1:0), CrossPANI/P-AC (1:0.2), and DB-86-CrossPANI/P-AC (1:0.2). Figure 4a shows FTIR of P-AC, the peaks at 3852–3707 and 3195 cm–1 representing bonded and non-bonding –OH groups, respectively, on their surface [79]. The band of C = O at 1590 cm–1 is characteristic of aldehydes, ketones, quinone and carboxylated groups [80, 81]. Also, the band at 1190 cm–1 represented the C-O stretching vibrations, and the band at 886–756 cm–1 indicated H2PO4– and PO42– disulfide or aromatic structures [82]. Meanwhile, the band at 467 cm–1 indicates metal oxide or aromatic structures [65, 83]. CrossPANI/P-AC (1:0.2) shows similar transmittance bands to CrossPANI/P-AC (1:0). Figure 4b, c of CrossPANI/P-AC (1:0) and CrossPANI/P-AC (1:0.2) shows the main characteristic peak positions of PANI; the broadband at ~ 3223.52 and 3372.92 cm−1, respectively, is attributed to the N–H stretching vibration due to the protonation of nitrogen [84, 85]; and the characteristic band at ~ 2966.29 and 2930.89 cm−1, respectively, can be assigned to the stretching vibration of the methyl group (–CH3). The stretching vibrations of the quinoid and benzenoid rings are represented by the two bands at 1591.06, 1593.86 cm–1 and 1499.75–1492.75 cm–1, respectively. The C–N = stretching vibration between benzenoid and quinoid units in CrossPANI/P-AC(1:0) is responsible for the peak at 1384.22 cm–1 [84, 86]. The band at ~ 1291.33, 1294.13 cm−1 and ~ 820.81, 814.95 cm–1, respectively, can be assigned for the stretching of C–N and bending of C–H (out of plane) in the benzene ring [87]. The peaks at ~ 1054.59 and 1060.50 cm–1 are related to the S = O and S–O stretching vibrations of the sulfonate groups linked to the aromatic rings, indicating that the produced PANI nanostructures were doped [84]. FTIR spectra of CrossPANI/P-AC (1:0.2) (Fig. 4d) after adsorption of DB-86 dye showed the same characteristic peak of CrossPANI/P-AC (1:0.2) with a small shift in peaks, and its intensity due to adsorption of DB-86 dye, indicating that the adsorption process occurred.
XRD analysis
Figure 5 shows the X-ray diffraction of P-AC, CrossPANI/P-AC(1:0), and CrossPANI/P-AC(1:0.2). The XRD pattern of P-AC showed characteristic diffraction peaks of crystalline structures of the graphitic-activated carbon. The presence of a sharp peak around 2θ = 21.14°, 26°, and 42° was due to the formation of a disordered graphitic phase [88,89,90]. The diffraction patterns of CrossPANI/P-AC(1:0) consisted of broad crystalline peaks; the prominent diffracted peaks at an angle of 2θ = 17.114°, 20.740°, 24.715°, respectively, due to the repeating of benzenoid and quinoid rings in PANI chains, poor crystallinity for conductive polymers are ascribed to the polymer chain, showing low crystallinity for conductive polymers [46, 51, 91, 92]. According to Ali et al. [93], new PANI peaks occurred at 2θ = 43.822°, 49.958°, and 63.974° due to new arrangements or cross-linking in the PANI structure. The CrossPANI/P-AC(1:0.2) peaks were found to slightly shift than CrossPANI/P-AC(1:0) peaks to 16.208, 20.88, 25.064, 43.682, 50.307, 63.974. The gross intensity of the peaks was raised following the addition of P-AC nanoparticles to the PANI matrix due to an interaction between P-AC nanoparticles and PANI caused by the creation of hydrogen bonding between H–N and oxygen of P-AC.
Pore structure analysis
Figure 6A shows the adsorption–desorption isotherms of nitrogen at 77 K on CrossPANI/P-AC(1:0), CrossPANI/P-AC(1:1), CrossPANI/P-AC(1:0.2), and CrossPANI/P-AC(1:0.6). CrossPANI/P-AC(1:0) and CrossPANI/P-AC(1:0.6) exhibit type-V isotherm with H3 hysteresis loop; this implies containing slit-like pores. The composites CrossPANI/P-AC(1:1) and CrossPANI/P-AC(1:0.2) show changes in N2 adsorption–desorption isotherm to type-IV with remarkable hysteresis loops according to the IUPAC classification [94]. This implies that the pores are mostly mesoporous and macroporous.
Furthermore, Fig. 6b shows BJH desorption pore size distributions of CrossPANI/P-AC(1:0), CrossPANI/P-AC(1:1), CrossPANI/P-AC(1:0.2), and CrossPANI/P-AC(1:0.6). It can be noted that the pores between 2 and 30 nm were dominant for all; that is, both mesopores and macropores are present. The porous structure parameters of CrossPANI/P-AC(1:0), CrossPANI/P-AC(1:1), CrossPANI/P-AC(1:0.2), and CrossPANI/P-AC(1:0.6) from the basis of the nitrogen adsorption data are summarized in Table 3. BET surface area and monolayer volume of CrossPANI/P-AC(1:0) reach 29.38 m2/g and 6.7503 cm3/g, respectively, which increased after the addition of P-AC to 166.10 m2/g and 38.163 cm3/g in CrossPANI/P-AC(1:0.2) and then decreased to 21.59 m2/g and 4.9593 cm3/g in CrossPANI/P-AC(1:0.6). The mean pore diameter of samples ranges 2–50 nm, meaning that samples' pore size is in mesopore.
Thermal analysis
The TGA/DTA thermograms of CrossPANI/P-AC(1:0) and CrossPANI/P-AC(1:0.2) were examined in the temperature range of 30 to 900 °C at a heating rate of 10 °C min–1 under a nitrogen atmosphere. Figure 7a,b indicates TGA/DTA plots of the decomposition of CrossPANI/P-AC(1:0) and CrossPANI/P-AC(1:0.2). The first stage involves water evaporation with approximately weight loss of 4.637, 4.293%, and an exothermic peak of 81.19, 84.30 °C is found in the DTA curve, respectively [93]. The second weight-loss stage had an exothermic peak at 290.62 and 284.29 °C, respectively, and a weight loss of 12.22 and 1.84 percent. This is due to the loss of amine groups as low molecular weight polymers degrade (NH2) [61]. In the third weight-loss stage for CrossPANI/P-AC(1:0), the weight loss was 23.56 with two exothermic peaks at 568.43 and 753.97 °C; for CrossPANI/P-AC(5:1) the weight loss was 66.66% with an exothermic peak at 644.29 °C due to thermal degradation of low molecular weight of PANI chains, loss of amine groups (NH2) and the elimination of P-AC [51]. The CrossPANI/P-AC(1:0.2) also shows the same stages of weight loss of CrossPANI/P-AC(1:0), with higher weight loss in the third stage as compared to CrossPANI/P-AC(1:0) due to the incorporation of P-AC in PANI matrix and a weight loss of 66.66% was observed. On examining TGA curves, it is observed that CrossPANI/P-AC(1:0) has higher thermal stabilities than CrossPANI/P-AC(1:0.2), as indicated by the decreased weight loss.
Points of zero charge PZCs
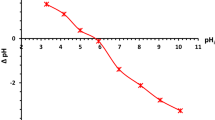
The point of zero charges is the pH required to affect a net zero charge on a solid surface without particular sorption (pHPZC). The adsorbent CrossPANI/P-AC(1:0.2) surface may exhibit positive and negative surface charges at pH < 3.15 and pH > 3.15, respectively, as presented in Fig. 8. From these values, it can be assumed that when immersed in a solution with a pH below 3.15, the composite surface will be positively charged, thus favoring the adsorption of anionic specimens. When the pH is greater than 3.15, the surface becomes negatively charged, facilitating the adsorption of cationic materials.
Adsorption behavior toward DB-86 dye
The removal % of DB-86 dye on CrossPANI/P-AC(1:0.2) with different ratios
The removal % of DB-86 dye on CrossPANI/P-AC with different ratios (1:0, 1:0.2, 1:0.6, 1:1) was tested at DB-86 dye of 50 mg/L, pH = 2.2, dose of 50 mg, contact time of 180 min, and shacking speed 200 rpm. Figure 9 shows that CrossPANI/P-AC with different ratios (1:0, 1:0.2, 1:0.6, 1:1) affects B-86 dye adsorption capacity as calculated using Eq. 1. The maximum DB-86 dye adsorption capacity obtained by CrossPANI/P-AC(1:0.2) reached 83.69 mg/g and was selected to perform the batch experimental. It can be noticed that the addition of P-AC to PANI increased DB-86 dye removal% at 0.2 dose and then decreased removal % with an increase dose than 0.2 dose. This can be explained by increased P-AC, blocking the adsorption sites on the PANI surface.
Influence of pH
The adsorption of DB-86 dye on CrossPANI/P-AC(1:0.2) as a function of pH at [DB-86 dye = 50 mg/L, dose = 50 mg, temperature = 25 ± 2 C, shacking speed = 200 rpm, contact time = 180 min] decreases with the increase in pH where the maximum capacity reaches 83.69 mg/g at pH 2.2 and the minimum adsorption capacity reaches 15.48 mg/g at both pH 7.3 and 8.5 (Fig. 10). That may be attributed to the point of zero charge (pHPZC) of CrossPANI/P-AC(1:0.2) surface, which is equal to 3.15. This leads to assuming that when CrossPANI/P-AC(1:0.2) is immersed in a DB-86 dye solution with pH below 3.15, the composite surface will be positively charged, thus favoring the adsorption of anionic specimens of DB-86 dye. Because the surface of CrossPANI/P-AC(1:0.2) is negatively charged, adsorption decreases as pH rises, creating conflict between anionic DB-86 dye and excess OH ions in the solution. The adsorption of DB-86 dye on activated carbon produced from orange peel [95], shrimp chitosan [96], and alginate-encapsulated activated carbon (PnsAC-alginate) [97] followed a similar trend of pH impact.
Influence of initial concentration
The adsorption experiments at initial DB-86 dye concentrations from 50 to 150 mg/L were performed with CrossPANI/P-AC(1:0.2) doses (0.5, 0.75, 1.0, 1.25 and 1.5 g/L), and the results are represented in Fig. 11. The results indicated that the percentage removal of DB-86 dye adsorbed on CrossPANI/P-AC(1:0.2) was inversely proportional to DB-86 dye concentration. The percentage of DB-86 dye removed was higher at lower initial DB-86 dye concentrations and smaller at higher initial concentrations, indicating that DB-86 dye adsorption from its aqueous solution was dependent on its initial concentration and that increasing DB-86 dye concentration reduced active surface size [80]. The percentage removal of DB-86 dye at dose 0.5 g/L, 1.5 g/L decreased from 83.69 to 56.83 and 97.14 to 91.11% when the initial DB-86 dye concentration increased from 50 to 150 mg/L, respectively. On the other hand, the maximum adsorption capacity at doses of 0.5 g/L and 1.5 g/L increased from 38.69 to 170.48 mg/g and 32.38 to 91.11 mg/g, respectively, when the initial DB-86 dye concentration increased from 50 to 150 mg/L.
Effect of contact time
Figure 12 shows the effect of contact time on the adsorption capacity of DB-86 dye by CrossPANI/P-AC(1:0.2) at different shaking times at concentrations 50, 75, 100, 125 and 150 mg/L. The adsorption capacity of DB-86 dye increases with the increase of the adsorption time and initial concentration, where the adsorption equilibrium was basically achieved within 90 min for an initial concentration of 50–150 mg/L for DB-86 dye.
Effect of CrossPANI/P-AC(1:0.2) dose
The effect of the adsorbent CrossPANI/P-AC(1:0.2) dose on DB-86 dye adsorption was performed by varying the dose from 0.5 to 1.5 g/L and different concentrations of DB-86 dye varying from 50–150 mg/L at pH 2.2 (Fig. 13). It is observed that the increase of the amount of the CrossPANI/P-AC(1:0.2) resulted in an increase of DB-86 dye removal with a maximum of 97.14% at 50 mg/L DB-86 dye, and so decreased the equilibrium adsorption capacity (qe) of DB-86 dye. These results are because greater sorbent dosages offer more sorbent functional groups, surface area, and pores volume accessible for DB-86 dye adsorption on the CrossPANI/P-AC(1:0.2) surface.
Adsorption kinetics
The kinetic adsorption data of DB-86 dye onto CrossPANI/P-AC(1:0.2) were processed to understand and predict the nature of the adsorption process. Table 1 summarizes the five linear equations of kinetic models employed to understand the reaction mechanism. Figure 14 and Table 4 display the pseudo-first-order and pseudo-second-order kinetics model of DB-86 adsorption onto CrossPANI/P-AC(1:0.2) parameters values. Data showed that in the pseudo-first-order kinetic model, the qe(exp) values are not in agreement with the predicted qe(th) values but they are in agreement with predicted qe(th) values of pseudo-second-order with R2 values greater than 0.993. The rate constant of pseudo-second-order adsorption increased with increase DB-86 dye concentration and CrossPANI/P-AC(1:0.2) doses. Therefore, the pseudo-second-order kinetic model is more adequate to describe the adsorption process of DB-86 dye onto CrossPANI/P-AC(1:0.2). Meanwhile, Fig. 14 and Table 5 display parameter values of Elovich, intraparticle diffusion, and liquid film diffusion model of DB-86 dye adsorption onto CrossPANI/P-AC(1:0.2). Results showed that the R2 values were ranged from (0.959–0.999), (0.909–0.991), and (0.912—0.998), respectively, for Elovich, intraparticle diffusion and liquid film diffusion model. In Elovich model, the initial sorption rate α has a wavy and identified role with DB-86 dye concentration and adsorbent dose. The desorption constant, β, decreases with increasing the initial concentration of DB-86 dye while increasing with increasing adsorbent dose. The results do not accord with the initial sorption rate, h, calculated using the pseudo-second-order model, indicating that this model is unsuitable for the experimental data obtained for the adsorption of DB-86 dye on CrossPANI/P-AC (1:0.2). The intraparticle diffusion plots did not pass through the origin, indicating that intraparticle diffusion was not the only rate-controlling step, although it was involved in the process. The Kdif and C values are directly proportional with DB-86 dye concentration and inversely proportional with CrossPANI/P-AC(1:0.2) doses. The film diffusion plots are linear, but they do not pass through the origin, explaining how the film diffusion mechanism affects the adsorption rate. With DB-86 dye concentration and CrossPANI/P-AC(1:0.2) dosages, the values of KFD and C play a wavy and indefinite role.
Adsorption isotherm
To describe the experimental result of DB-86 dye removal isotherm on CrossPANI/P-AC(1:0.2) composite, four isotherm models, which are Langmuir, Freundlich, Tempkin, and Dubinin–Radushkevich (D–R) isotherms, have been investigated. The equations representing these models are compiled in Table 2. Fig. 15 and Table 6 illustrate each linearized isotherm model's plots and related parameters. However, the arrangement of adsorptive removal of DB-86 dye by CrossPANI/P-AC(1:0.2) isotherm as Langmuir < Dubinin-Radushkevich < Tempkin< Freundlich based on the coefficient of determination values (R2) and the low value of APE% error model.
According to the Langmuir model, DB-86 dye adsorption occurs on a homogenous surface via monolayer adsorption, without interaction between adsorbed species [98,99,100]. The maximum monolayer capacity (Qm) was 163.93 mg/g at dose 0.5 g/L. The separation factor RL is calculated using the following equation: RL = 1/(1 + KaCo) [33, 83]. It was found that the adsorption process of DB-86 dye onto CrossPANI/P-AC(1:0.2) is a favorable process. Meanwhile, Freundlich isotherm suggested the adsorption process of DB-86 dye based on adsorption heterogeneous surface. The degree of nonlinearity between solution concentration and adsorption is indicated by the n value [68]. Results showed that the adsorption process is a favorable physical process where the value of n < 1. According to the Tempkin isotherm model, the heat of adsorption of all molecules in the layer declines linearly with coverage due to adsorbate–adsorbate interactions, and adsorption is characterized by a uniform distribution of binding energies up to some maximum binding energy. Table 6 shows the values of the Tempkin isotherm parameters. D–R isotherm was also applied to estimate the porosity of apparent free energy and the characteristics of adsorption. It may be used to explain adsorption on both homogenous and heterogeneous surfaces [68, 101]. The maximum capacity Qm obtained for DB-86 adsorption is 158.63 mg/g, less than the Qm obtained using the Langmuir isotherm model. The computed E values range from 0.1055 to 0.9129 kJ/mol, with values less than 8 kJ/mol, showing that the physical-sorption process is important in the adsorption of DB-86 dye onto CrossPANI/P-AC(1:0.2).
Thermodynamic study
The removal of DB-86 dye onto CrossPANI/P-AC(1:0.2) as a function of reaction temperature was estimated in the temperature range 25–50 °C and is represented in Fig. 16. The capacity of DB-86 dye onto CrossPANI/P-AC(1:0.2) was directly proportional to temperature. The adsorptive removal processes of DB-86 dye are proceeding in endothermic reactions. The maximum DB-86 adsorption capacity was 94.76 mg/g using the initial concentration of solution 50 mg/L at about 50 °C, pH 2.2 and 0.5 g/L adsorbent dosage.
According to Table 7, all ∆G° values were negative and increased with increasing temperature, indicating that the DB-86 dye adsorption process on CrossPANI/P-AC(1:0.2) was spontaneous suggesting stronger adsorptive forces between adsorbent and adsorbate. The positive ∆H° value (13.720 kJ/mol) of DB-86 dye adsorption on CrossPANI/P-AC(1:0.2) confirmed the involvement of an endothermic process. The enthalpy changes ΔH° were less than 84 kJ/mol, indicating that the physisorption adsorption process was taking place [102, 103]. The entropy (∆S°) value was also positive (65.05 J/K mol), which explains the enhanced randomness at the solid/solution interface during the adsorption of DB-86 dye onto CrossPANI/P-AC(1:0.2).
Mechanism of adsorption
The chemical structure of the adsorbents influences the adsorption phenomena. The following steps in order could be used to describe the adsorption mechanism of DB-86 dye onto CrossPANI/P-AC(1:0.2): (i) through a boundary layer, DB-86 dye is transported from a bulk solution to the external surface of CrossPANI/P-AC(1:0.2) (liquid film diffusion); (ii) as illustrated in Fig. 17, dye adsorption on the adsorbent surface may be owing to the establishment of weak hydrogen bonds between the positively charged CrossPANI/P-AC(1:0.2) and the oxygen atoms of DB-86 dye molecules. At the solid/liquid contact, the following reactions may have occurred:
CrossPANI/P-AC(1:0.2) + H+ → (CrossPANI/P-AC(1:0.2))H+
(CrossPANI/P-AC(1:0.2))H+ + DB-86– → (CrossPANI/P-AC(5:1))H+….DB-86–
(Electrostatic interaction causes the formation of a weak hydrogen bond)
(iii) DB-86 dye transport from the exterior to the pores of CrossPANI/P-AC (1:0.2) (intraparticle diffusion); then, (iv) DB-86 dye adsorption on the active site in the inner and outer surface of the CrossPANI/P-AC (1:0.2).
Desorption and regeneration studies
Desorption experiments of DB-86 dye from the loaded CrossPANI/P-AC(1:0.2) were performed using 0.1 N NaOH as an eluted medium to investigate the economic feasibility and reusability CrossPANI/P-AC(1:0.2) adsorbents. In this condition, the desorption % decreased with increased regeneration cycles, as shown in Fig. 18a. The regenerated CrossPANI/P-AC(1:0.2) composite was applied in three consecutive cycles of adsorption/desorption. The adsorption amount presented was consistent through the cycles and experienced the adsorption capacity decreased by 24.29 mg/g after third generation, which suggests it may be used as a sustainable DB-86 dye removal (Fig. 18b).
Comparative adsorption capacities of different adsorbents for DB-86 dye
The adsorption capacity of CrossPANI/P-AC(1:0.2) composite was compared with some previously reported adsorbents (Table 8).
Conclusion
The in situ polymerization method was used to synthesize crosslinked polyaniline /Pterocladia capillacea—activated carbon composites (CrossPANI/P-AC) at different ratios (1:0, 1:0.2, 1:0.6, and 1:1). The samples were characterized by FTIR, BET, XRD, TGA, SEM, and EDX. Analysis showed that the main characteristic peak positions of CrossPANI/P-AC by FTIR, low crystallinity nature by XRD, 166.10 m2/g a high specific surface area for CrossPANI/P-AC(1:0.2) were achieved by BET, amorphous nature and polymerization occurred at the surface of activated carbon by SEM. The performance of CrossPANI/P-AC (1:0.2) composite on the DB-86 dye removal from aqueous solution was reviewed in detail. The pH of the reaction was discovered to play a significant influence in the DB-86 dye removal process, with pH 2.2 being the ideal pH for DB-86 dye removal. The maximum monolayer capacity (Qm) was 163.93 mg/g at dose 0.5 g/L. The results for DB-86 dye sorption fit the Langmuir isotherm model better than the Freundlich model. The values (ΔG°, ΔH°, and ΔS°) of thermodynamic parameters support that the DB-86 dye adsorption by CrossPANI/P-AC(1:0.2) composite was physical adsorption, endothermic, and spontaneous in nature. The kinetic data were then fitted into a pseudo-second-order model. Based on these findings, the CrossPANI/P-AC(1:0.2) composite can be considered a promising adsorbent for effectively removing DB-86 dye ions from industrial effluent while also being recyclable.
References
Rizzi V, Longo A, Fini P, Semeraro P, Cosma P, Franco E, Ferrándiz M (2014) Applicative study (part I): the excellent conditions to remove in batch direct textile dyes (direct red, direct blue and direct yellow) from aqueous solutions by adsorption processes on low-cost chitosan films under different conditions. Adv Chem Eng Sci 4(04):454. https://doi.org/10.4236/aces.2014.44048
West, Larry (March 26, 2006). "World water day: a billion people worldwide lack safe drinking water"
El Nemr A (2011) Impact, monitoring and management of environmental pollution” Nova Science Publishers, Inc. Hauppauge New York. [ISBN-10: 1608764877, ISBN-13: 9781608764877] 638 pages.
El Nemr A (2012) Environmental pollution and its relation to climate change. Nova Science Publishers, Inc. Hauppauge New York. [ISBN-13: 978–1–61761–794–2] 694 pages.
Kelland, Kate (October 19, 2017). "Study links pollution to millions of deaths worldwide
El Nemr A (2012) Textiles: Types, uses and production methods. Nova Science Publishers, Inc. Hauppauge New York. Hard cover [ISBN: 978–1–62100–239–0], e-book [ISBN: 978–1–62100–284–0] 621 pages.
Hassaan MA, El Nemr A, Madkour FF (2017) Testing the advanced oxidation processes on the degradation of direct blue 86 dye in wastewater. Egypt J Aquat Res 43(1):11–19. https://doi.org/10.1016/j.ejar.2016.09.006
Kalotra S, Mehta R (2021) Synthesis of polyaniline/clay nanocomposites by in situ polymerization and its application for the removal of acid green 25 dye from wastewater. Polym Bull 78(5):2439–2463. https://doi.org/10.1007/s00289-020-03222-3
Hassaan MA, El Nemr A (2017) Health and environmental impacts of dyes: mini review, American. J Environ Sci Eng 1(3):64–67. https://doi.org/10.11648/j.ajese.20170103.11
http://www.worlddyevariety.com/direct-dyes/direct-blue-86.html; https://emperordye.com/tds/direct-blue-86.html; https://pubchem.ncbi.nlm.nih.gov/compound/Direct-blue-86
Dutta M, Basu JK, Faraz MH, Gautam N, Kumar A (2012) Fixed–bed column study of textile dye direct blue 86 by using a composite adsorbent. Arch Appl Sci Res 4(2):882–891
El Nemr A (2012) Non-conventional textile waste water treatment. Nova Science Publishers, Inc. Hauppauge New York. [Hard cover ISBN: 978–1–62100–079–2, e-book ISBN: 978–1–62100–228–4] 267 pages.
Zhuang J, Qi Y, Yang H, Li H, Shi T (2021) Preparation of polyaluminum zirconium silicate coagulant and its performance in water treatment. J Water Process Eng 41:102023. https://doi.org/10.1016/j.jwpe.2021.102023
Chan MK, Abdullah N, Rageh EHA, Kumaran P, Tee YS (2021) Oxidation of ammonia using immobilised FeCu for water treatment. Sep Purif Technol 254:117612. https://doi.org/10.1016/j.seppur.2020.117612
Hassaan MA, El Nemr A, Madkour FF (2016) Application of ozonation and UV assisted ozonation for decolorization of direct yellow 50 in sea water. Pharma Chem J 3(2):131–138
Hassaan MA, El Nemr A, Madkour FF (2017) Advanced oxidation processes of mordant violet 40 dye in freshwater and seawater. Egypt J Aquat Res 43:1–9. https://doi.org/10.1016/j.ejar.2016.09.004
Hassan MA, El Nemr A (2017) advanced oxidation processes for textile wastewater treatment. Int J Photochem Photobiol 2(3):85–93
Hassaan MA, El Nemr A, El-Zahhar AA, Idris AM, Alghamdi MM, Sahlabji T, Said TO (2020) Degradation mechanism of Direct Red 23 dye by advanced oxidation processes: a comparative study. Toxin Rev. https://doi.org/10.1080/15569543.2020.1827431
El Nemr A, Madkour MA (2017) HPLC-MS/MS mechanistic study of direct yellow 12 degradation using ultraviolet assisted ozone process. J Water Environ Nanotechnol 3(1):1–11. https://doi.org/10.22090/jwent.2018.01.001
El Nemr A, Hassaan MA, Madkour FF (2018) Advanced oxidation process (AOP) for detoxification of acid red 17 dye solution and degradation mechanism. Environ Process 5(1):95–113. https://doi.org/10.1007/s40710-018-0284-9
Helmy ET, El Nemr A, Mousa M, Arafa E, Eldafrawy S (2018) Photocatalytic degradation of organic dyes pollutants in the industrial textile wastewater by using synthesized TiO2, C-doped TiO2, S-doped TiO2 and C, S co-doped TiO2 nanoparticles. J Water Environ Nanotechnol 3(2):116–127. https://doi.org/10.22090/jwent.2018.02.003
Helmy ET, El Nemr A, Gomaa E, El Dafrawy S, Mousa M (2021) Photocatalytic degradation of textile dyeing wastewater under visible light irradiation using green synthesized mesoporous non-metal doped TiO2. Bull Mater Sci 44:30. https://doi.org/10.1007/s12034-020-02322-0
El Nemr A, Helmy ET, Arafa E, Eldafrawy S, Mousa M (2019) Photocatalytic and biological activities of undoped and doped TiO2 prepared by green method for water treatment. J Environl Chem Eng 7(5):103385. https://doi.org/10.1016/j.jece.2019.103385
Hassaan MA, Elkatory MR, El Nemr A (2020) Applications of photochemical oxidation in textile industry. Book chapter, Springer, Handbook of nanomaterials and nanocomposites for energy and environmental applications. https://doi.org/10.1007/978-3-030-11155-7_59-1
Hassaan MA, Elkatory MR, Ali RM, El Nemr A (2020) Photocatalytic degradation of reactive black 5 using photo-Fenton and ZnO nanoparticles under ultraviolet light irradiation. Egyptn J Chem 63(4):17–18
Kohansal S, Haghighi M, Zarrabi M (2021) Intensification of Bi7O9I3 nanoparticles distribution on ZnO via ultrasound induction approach used in photocatalytic water treatment under solar light irradiation. Chem Eng Sci 230:116086. https://doi.org/10.1016/j.ces.2020.116086
Chanikya P, Nidheesh PV, Babu DS, Gopinath A, Kumar MS (2021) Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep Purif Technol 254:117570. https://doi.org/10.1016/j.seppur.2020.117570
Aboudalle A, Djelal H, Domergue L, Fourcade F, Amrane A (2021) A novel system coupling an electro-Fenton process and an advanced biological process to remove a pharmaceutical compound, metronidazole. J Hazard Mater 415:125705. https://doi.org/10.1016/j.jhazmat.2021.125705
Rajaniemi K, Hu T, Nurmesniemi ET, Tuomikoski S, Lassi U (2021) Phosphate and ammonium removal from water through electrochemical and chemical precipitation of struvite. Processes 9(1):150. https://doi.org/10.3390/pr9010150
El-Nemr MA, Ismail IMA, Abdelmonem NM, Ragab S, El Nemr A (2020) Ozone and ammonium hydroxide modification of biochar prepared from pisum sativum peels improves the adsorption of copper (II) from an aqueous medium. Environ Process 7:973–1007. https://doi.org/10.1007/s40710-020-00455-2
El-Nemr MA, Abdelmonem NM, Ismail IMA, Ragab S, El Nemr A (2020) The efficient removal of the hazardous Azo Dye Acid orange 7 from water using modified biochar from Pea peels. Desalin Water Treat 203:327–355. https://doi.org/10.5004/dwt.2020.26190
El-Nemr MA, Abdelmonem NM, Ismail IMA, Ragab S, El Nemr A (2020) Removal of acid yellow 11 Dye using novel modified biochar derived from watermelon peels. Desalin Water Treat 203:403–431. https://doi.org/10.5004/dwt.2020.26207
El-Nemr MA, Ismail IMA, Abdelmonem NM, El Nemr A, Ragab S (2020) Amination of biochar derived from Watermelon peel by Triethylenetetramine and ammonium hydroxide for toxic chromium removal enhancement. Chin J Chem Eng In press
El Nemr A, Shoaib AG, El Sikaily A, Mohamed AEDA, Hassan AF (2021) Evaluation of cationic methylene blue dye removal by high surface area mesoporous activated carbon derived from Ulva lactuca. Environ Process 8(1):311–332. https://doi.org/10.1007/s40710-020-00487-8
Sahlabji T, El-Nemr MA, El Nemr A, Ragab S, Alghamdi MM, El-Zahhar AA, Idris AM, Said TO (2021) High surface area microporous activated carbon from Pisum sativum peels for hexavalent chromium removal from aquatic environment. Toxin Revi 41(2):639–649. https://doi.org/10.1080/15569543.2021.1908361
El Nemr A, Aboughaly RM, El Sikaily A, Ragab S, Masoud MS, Ramadan MS (2020) Microporous nano-activated carbon type I derived from orange peel and its application for Cr(VI) removal from aquatic environment. Biomass Convers Biorefine. https://doi.org/10.1007/s13399-020-00995-5
Eleryan A, El Nemr A, Mashaly M, Khaled A (2019) 6-Triethylenetetramine 6-deoxycellulose grafted with crotonaldehyde as adsorbent for Cr6+ removal from wastewater. Int J Sci Eng Res 10(7):1199–1211
Soto ML, Moure A, Domínguez H, Parajó JC (2011) Recovery, concentration and purification of phenolic compounds by adsorption: a review. J Food Eng 105(1):1–27. https://doi.org/10.1016/j.jfoodeng.2011.02.010
Eldeeb TM, El Nemr A, Khedr MH, El-Dek SI, Imam NG (2020) Novel three-dimensional chitosan-carbon nanotube–PVA nanocomposite hydrogel for removal of Cr6+ from wastewater. Desalin Water Treat 184:163–177. https://doi.org/10.5004/dwt.2020.25366
Serag E, El Nemr A, Fathy SA, Abdel Hamid F, El-Maghraby A (2018) A novel three dimensional carbon Nanotube-polyethylene glycol-Polyvinyl alcohol Nanocomposite for Cu (II) removal from water. Egypt J Aquatic Biol Fish 22(2):103–118. https://doi.org/10.21608/EJABF.2018.8234
Shoaib AGM, El-Sikaily A, El Nemr AE, Hassan AF (2020) Preparation and characterization of highly surface area activated carbons followed type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Convers Biorefine. https://doi.org/10.1007/s13399-020-00760-8
Yin W, Ruckenstein E (2000) Soluble polyaniline co-doped with dodecyl benzene sulfonic acid and hydrochloric acid. Synth Met 108(1):39–46. https://doi.org/10.1016/S0379-6779(99)00179-4
Shao L, Qiu J, Liu M, Feng H, Lei L, Zhang G, Qin L (2011) Synthesis and characterization of water-soluble polyaniline films. Synth Met 161(9–10):806–811. https://doi.org/10.1016/j.synthmet.2011.02.003
Karci F, Kabasakaloglu M (2013) Ion exchange and corrosion behaviour of polyaniline coated mild steel electrode. Asian J Chem 25(5):2533–2536
Ju Q, Huang H, Kan J (2013) Treatment of chemically synthesized polyaniline wastewater by combining adsorption of activated carbon and neutralization of calcium carbonate. Asian J Chem 25(17):9543–9546
Morsi RE, Elsabee MZ (2015) Polyaniline nanotubes: mercury and competative heavy metals uptake. Am J Polym Sci 5(1):10–17. https://doi.org/10.5923/j.ajps.20150501.02
Zhou T, Li C, Jin H, Lian Y, Han W (2017) Effective adsorption/reduction of Cr (VI) oxyanion by halloysite@ polyaniline hybrid nanotubes. ACS Appl Mater Int 9(7):6030–6043
Kong P, Liu P, Ge Z, Tan H, Pei L, Wang J, Li Z (2019) Conjugated HCl-doped polyaniline for photocatalytic oxidative coupling of amines under visible light. Catal Sci Technol 9(3):753–761
Qiu B, Xu C, Sun D, Wang Q, Gu H, Zhang X, Wei S (2015) Polyaniline coating with various substrates for hexavalent chromium removal. Appl Surf Sci 334:7–14. https://doi.org/10.1016/j.apsusc.2014.07.039
Ansari R (2006) Application of polyaniline and its composites for adsorption/recovery of chromium (VI) from aqueous solutions. Acta Chim Slov 53(1):88–94
Ahmad R (2019) Polyaniline/ZnO nanocomposite: a novel adsorbent for the removal of Cr (VI) from aqueous solution. In: Advances in composite materials development. IntechOpen https://doi.org/10.5772/intechopen.85868
Gu H, Rapole SB, Sharma J, Huang Y, Cao D, Colorado HA, Guo Z (2012) Magnetic polyaniline nanocomposites toward toxic hexavalent chromium removal. RSC Adv 2(29):11007–11018
Harijan DK, Chandra V (2016) Polyaniline functionalized graphene sheets for treatment of toxic hexavalent chromium. J Environ Chem Eng 4(3):3006–3012. https://doi.org/10.1016/j.jece.2016.06.014
Karthik R, Meenakshi S (2015) Adsorption study on removal of Cr(VI) ions by polyaniline composite. Desalin Water Treat 54(11):3083–3093. https://doi.org/10.1080/19443994.2014.909330
Ren J, Huang X, Wang N, Lu K, Zhang X, Li W, Liu D (2016) Preparation of polyaniline-coated polyacrylonitrile fiber mats and their application to Cr(VI) removal. Synth Met 222:255–266. https://doi.org/10.1016/j.synthmet.2016.10.027
Wu H, Wang Q, Fei GT, Xu SH, Guo X, De Zhang L (2018) Preparation of hollow polyaniline micro/nanospheres and their removal capacity of Cr(VI) from wastewater. Nanoscale Res Lett 13:401. https://doi.org/10.1186/s11671-018-2815-8
Kanwal F, Rehman R, Mahmud T, Anwar J, Ilyas R (2012) Isothermal and thermodynamical modeling of chromium (III) adsorption by composites of polyaniline with rice husk and saw dust. J Chil Chem Soc 57(1):1058–1063. https://doi.org/10.4067/S0717-97072012000100022
Ghorbani M, Eisazadeh H, Ghoreyshi AA (2012) Removal of zinc ions from aqueous solution using polyaniline nanocomposite coated on rice husk. Iranica J Energ Environ 3(1):83–88
Mansour MS, Ossman ME, Farag HA (2011) Removal of Cd (II) ion from waste water by adsorption onto polyaniline coated on sawdust. Desalination 272(1–3):301–305. https://doi.org/10.1016/j.desal.2011.01.037
Bushra R, Shahadat M, Khan MA, Adnan R, Arshad M, Rafatullah M, Naushad M (2015) Preparation of polyaniline based nanocomposite material and their environmental applications. Int J Environ Sci Technol 12(11):3635–3642. https://doi.org/10.1007/s13762-014-0726-5
Mahmoud ME, Fekry NA, El-Latif MM (2016) Nanocomposites of nanosilica-immobilized-nanopolyaniline and crosslinked nanopolyaniline for removal of heavy metals. Chem Eng J 304:679–691. https://doi.org/10.1016/j.cej.2016.06.110
Geetha A, Palanisamy PN (2015) Kinetics and equilibrium studies on the removal of anionic dyes using polyaniline coated sawdust composite. Int J ChemTech Res 7(5):2439–2447
Lalitha G, Hemamalini R, Mu N (2018) Efficient photocatalytic degradation of toxic dyes using nanostructured TiO2/polyaniline nanocomposite. Desalin Water Treat 108:322–328. https://doi.org/10.5004/dwt.2018.21967
Zareyee D, Tayebi H, Javadi SH (2012) Preparation of polyaniline/activated carbon composite for removal of reactive red 198 from aqueous solution. Iran J Org Chem 4(1):799–802
Kumar R, Ansari MO, Barakat MA (2014) Adsorption of brilliant green by surfactant doped polyaniline/MWCNTs composite: evaluation of the kinetic, thermodynamic, and isotherm. Ind Eng Chem Res 53(17):7167–7175. https://doi.org/10.1021/ie500100d
Shoaib AG, El-Sikaily A, El Nemr A, Mohamed AEDA, Hassan AA (2020) Testing the carbonization condition for high surface area preparation of activated carbon following type IV green alga Ulva lactuca. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00823-w
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Sven Vetenskap Handl 24:1–39
El Nemr A, El-Sikaily A, Khaled A (2010) Modeling of adsorption isotherms of Methylene Blue onto rice husk activated carbon. Egypt J Aquat Res 36(3):403–425
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Srinivasan K, Balasubramanian N, Ramakrishan TV (1988) Studies on chromium removal by rice husk carbon. Indian J Environ Health 30:376–387
Boyed GE, Adamson AM, Myers LS (1949) The exchange adsorption of ions from aqueous solutions by organic Zeolites. J Am Chem Soc 69(11):2836–2848
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Freundlich H (1906) Über die adsorption in Lösungen. Z Phys Chem 57:385–470
Tempkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Phys URSS 12:217–222
Radushkevich LV (1949) Potential theory of sorption and structure of carbons. Zh Fiz Khim 23:1410–1420
Dubinin MM (1965) Modern state of the theory of volume filling of micropore adsorbents during adsorption of gases and steams on carbon adsorbents. Zh Fiz Khim 39:1305–1317
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266. https://doi.org/10.1021/cr60204a006
Krug R, Hunter W, Grieger R (1976) Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data. J Phys Chem A 80:2335–2341. https://doi.org/10.1021/j100562a006
El Sikaily A, El Nemr A, Khaled A (2011) Copper sorption onto dried red alga Pterocladia capillacea and its activated carbon. Chem Eng J 168:707–714. https://doi.org/10.1016/j.cej.2011.01.064
El Nemr A, El Sikaily A, Khaled A, Abdelwahab O (2015) Removal of toxic chromium from aqueous solution, wastewater and saline water by marine red alga Pterocladia capillacea and its activated carbon. Arab J Chem 8:105–117. https://doi.org/10.1016/j.arabjc.2011.01.016
Oliveira LCA, Pereira E, Guimaraes IR, Vallone A, Pereirac M, Mesquita JP, Sapag K (2009) Preparation of activated carbons from coffee husks utilizing FeCl3and ZnCl2 as activating agents. J Hazard Mater 165:87–94. https://doi.org/10.1016/j.jhazmat.2008.09.064
Kılıç M, Apaydın-Varol E, Pütün AE (2012) Preparation and surface characterization of activated carbons from Euphorbia rigida by chemical activation with ZnCl2, K2CO3, NaOH and H3PO4. Appl Surf Sci 261:247–254. https://doi.org/10.1016/j.apsusc.2012.07.155
El Nemr A, Shoaib AGM, El Sikaily A, Ragab S, Alaa E-D, Mohamed AF, Hassan, (2021) Utilization of green alga Ulva lactuca for sustainable production of meso-micro porous nano activated carbon for adsorption of Direct Red 23 dye from aquatic environment. Carbon Lett In press 02(06):2021. https://doi.org/10.1007/s42823-021-00262-1
Shao W, Jamal R, Xu F, Ubul A, Abdiryim T (2012) The effect of a small amount of water on the structure and electrochemical properties of solid-state synthesized polyaniline. Materials 5(10):1811–1825. https://doi.org/10.3390/ma5101811
Bhadra S, Singha NK, Khastgir D (2006) Polyaniline by new miniemulsion polymerization and the effect of reducing agent on conductivity. Synth Met 156(16–17):1148–1154. https://doi.org/10.1016/j.synthmet.2006.08.002
Wei Y, Hsueh KF, Jang GW (1994) A study of leucoemeraldine and effect of redox reactions on molecular weight of chemically prepared polyaniline. Macromolecules 27(2):518–525. https://doi.org/10.1021/ma00080a028
Emran KM, Ali SM, Al-Oufi AL (2017) Synthesis and characterization of nano-conducting copolymer composites: Efficient sorbents for organic pollutants. Molecules 22(5):772. https://doi.org/10.3390/molecules22050772
Baishya P, Maji TK (2018) A comparative study on the properties of graphene oxide and activated carbon based sustainable wood starch composites. Int J Biol Macromol 115:970–977. https://doi.org/10.1016/j.ijbiomac.2018.04.192
Kalagatur NK, Karthick K, Allen JA, Nirmal Ghosh OS, Chandranayaka S, Gupta VK, Krishna K, Mudili V (2017) Application of activated carbon derived from seed shells of Jatropha curcas for decontamination of zearalenone mycotoxin. Front Pharmacol 8:760. https://doi.org/10.3389/fphar.2017.00760
Shoaib AG, El Nemr A, El Sikaily A, Masoud MS, Ramadan MS (2022) Amidoxime modification of polyacrylonitrile/Pterocladia capillacea-derived activated carbon composite for adsorption of toxic chromium from aquatic environment. Carbon Letters 32:513–535
Wu H, Wang Q, Fei GT, Xu SH, Guo X, De Zhang L (2018) Preparation of hollow polyaniline micro/nanospheres and their removal capacity of Cr (VI) from wastewater. Nanoscale Res Lett 13:401. https://doi.org/10.1186/s11671-018-2815-8
Mahmoud ME, Saad EA, El-Khatib AM, Soliman MA, Allam EA, Fekry NA (2018) Green solid synthesis of polyaniline-silver oxide nanocomposite for the adsorptive removal of ionic divalent species of Zn/Co and their radioactive isotopes 65Zn/ 60Co. Environ Sci Pollut Res 25(22):22120–22135. https://doi.org/10.1007/s11356-018-2284-8
Ali SM, Emran KM, Al-Oufi AL (2017) Adsorption of organic pollutants by nano-conducting polymers composites: effect of the supporting nano-oxide type. J Mol Liq 233:89–99. https://doi.org/10.1016/j.molliq.2017.03.006
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid interface with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198254112201
El Nemr A, Abdelwahab O, El-Sikaily A, Khaled A (2009) Removal of direct blue-86 from aqueous solution by new activated carbon developed from orange peel. J Hazard Mater 161(1):102–110. https://doi.org/10.1016/j.jhazmat.2008.03.060
Fat’hi MR, Ahmadi A (2016) Dye removal from wastewater using shrimp chitosan as an eco-friendly adsorbent. Int J Environ Health Eng 5(1):19. https://doi.org/10.4103/2277-9183.190645
Garg D, Majumder CB, Kumar S, Sarkar B (2019) Removal of Direct Blue-86 dye from aqueous solution using alginate encapsulated activated carbon (PnsAC-alginate) prepared from waste peanut shell. J Environ Chem Eng 7(5):103365. https://doi.org/10.1016/j.jece.2019.103365
Kumarathilaka P, Jayaweera V, Wijesekara H, Kottegoda IRM, Rosa SRD, Vithanage M (2016) Insights into starch coated nanozero valent iron-graphene composite for Cr (VI) removal from aqueous medium. J Nanomater 2016:2813289. https://doi.org/10.1155/2016/2813289
Ismail MNM, El Nemr A, El Ashry ESH, Abdel Hamid H (2020) Novel simple modification of chitosan as adsorptive agent for removal of Cr6+ from aqueous solution. Egypt J Chem 63(4):1219–1240
Ismael MNM, El Nemr A, El Ashry ESH, Abdel Hamid H (2020) Removal of hexavalent chromium by cross-linking chitosan and N, N’-methylene Bis-acrylamide. Environ Process 7:911–930. https://doi.org/10.1007/s40710-020-00447-2
Shahwan T, Erten HN (2004) Temperature effects on barium sorption on natural kalinite and chlorite-illite clays. J Radioanal Nucl Chem 260(1):43–48. https://doi.org/10.1023/B:JRNC.0000027059.66424.b4
Faust SD, Aly OM, (1987) Adsorption processes for water treatment, Butterworth London
Assia Benhouria Md, Azharul Islam H, Zaghouane-Boudiaf M, Boutahala BHH (2015) Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chem Eng J 270:621–630. https://doi.org/10.1016/j.cej.2015.02.030
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was partially funded by the Science and Technology Development Fund (STDF) of Egypt (Projects No. CB-4874 and CB-22816).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shoaib, A.G.M., El Nemr, A., Ramadan, M.S. et al. Composite fabrication and characterization of crosslinked polyaniline/Pterocladia capillacea-activated carbon for adsorption of direct blue-86 dye from water. Polym. Bull. 80, 10393–10428 (2023). https://doi.org/10.1007/s00289-022-04563-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04563-x