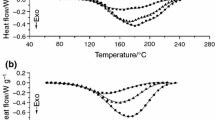
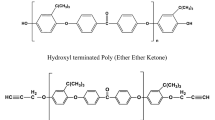
Abstract
Cardanol is a naturally occurring chemical compound consisting of meta substituted alkyl phenol. Two types of epoxy resins have been synthesized from cardanol. The synthesized epoxy resins have been characterized by FTIR and NMR spectroscopic analyses. The thermal stabilities and kinetics of the thermal degradation of cardanol-based resins were studied by thermogravimetric analysis under a nitrogen atmosphere with heating rates of 5, 10, 15 and 20 °C min−1. The molecular weights of the prepared novolac and epoxidized novolac resins were determined by gel permeation chromatography analysis. There is intense interest in understanding the degradation behavior and properties of cardanol-based epoxy resins. Three model-free methods, Kissinger, Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO), and the model-fitting Coats–Redfern method were employed to identify the kinetic triplet including activation energy, pre-exponential factor and reaction model. It was established that the Coats–Redfern model-fitting method is suitable for determining the kinetic reaction mechanism, but the most probable reaction functions R3 and D3 can be evaluated on the basis of the activation energy value which is nearest to the value of activation energy obtained by the FWO and KAS methods. A kinetic compensation effect was also observed for the above-mentioned bio-based epoxy resins.
















Similar content being viewed by others
References
Murthy BGK, Sivasamban MA (1985) Recent trends in CNSL utilization. Acta Hort (ISHS) Acta Hortic 108:201–207
Mwaikambo LY, Ansell MP (2001) Cure characteristics of alkali catalyzed cashewnut shell liquid formaldehyde resin. J Mater Sci 36:3693–3698
Bisanda ETN, Ogola WO, Tesha JV (2003) Characterisation of tannin resin blends for particleboard applications. Cement Concr Compos 25:593–598
Biswas BK, Biswas S, Khan M, Chandra Ray B (2009) Preparation and characterization of CNSL modified phenol formaldehyde resin. J Polym Mater 28:7–15
Devi A, Srivatsava D (2007) Studies on the blends of cardanol-based epoxidized novolac type phenolic resin and carboxyl-terminated polybutadiene (CTPB), I. Mat Sci Eng A 458:336–347
Yadav R, Srivatsava D (2010) Blends of cardanol-based epoxidized novolac resin and CTBN for application in surface coating: a study on thermal, mechanical, chemical, and morphological characteristics. J Coat Technol Res 7:557–568
Sultania M, Srivatsava D (2011) The effect of CTBN concentrations on the kinetic parameters of decomposition of blends of epoxy resins modified with carboxyl-terminated liquid copolymer. J Polym Environ 19:950–956
Mythili CV, Malar Retna A, Gopalakrishnan S (2004) Synthesis, mechanical, thermal and chemical properties of polyurethanes based on cardanol. Bull Mater Sci 27:235–241
Mythili CV, Malar Retna A, Gopalakrishnan S (2005) Physical, mechanical, and thermal properties of polyurethanes based on hydroxyalkylated cardanol–formaldehyde resins. J Appl Polym Sci 98:284–288
Manjula S, Pavithran C, Pillai CKS, Kumar VG (1991) Synthesis and mechanical properties of cardanol–formaldehyde (CF) resins and CF-poly(methylmethacrylate) semi-interpenetrating polymer networks. J Mater Sci 26:4001–4007
Manjula S, Pillai CKS, Kumar VG (1990) Thermal characterization of cardanol–formaldehyde resins and cardanol–formaldehyde/poly(methyl methacrylate) semi-interpenetrating polymer networks. Thermochim Acta 159:255–266
Chen YF, Chen ZB, Xiao SY, Liu HB (2008) A novel thermal degradation mechanism of phenol–formaldehyde type resins. Thermochim Acta 47:639–643
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Popescu C (1996) Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions A variant on the Ozawa–Flynn–Wall method. Thermochim Acta 285:309–323
Çetin NS, Özmen N (2002) Use of organosolv lignin in phenol–formaldehyde resins for particleboard production I. Organosolv lignin modified resins. Int J Adhes Adhes 22:477–480
Perin DD, Armerigo WLF (1988) Purification of laboratory chemicals. Pergamon, New York
Sorenson WR, Campbell TW (1968) Preparative methods of polymer chemistry. Wiley-interscience, New York
Gedam PH, Sampathkumaram PS, Sivasamban MA (1972) Examination of the components of cashew nut shell liquid by NMR. Indian J Chem 10:388–391
Murthy BGK, Sivasamban MA, Agarwal JS (1968) Identification of some naturally occurring alkyl-substituted phenols in cashew-nut shell liquid by chromatographic techniques. J Chromatogr 32:519–528
Lee H, Neville K (1967) Handbook of epoxy resins. McGraw-Hill, New York
Suresh KI, Kishanprasad VS (2005) Synthesis, structure, and properties of novel polyols from cardanol and developed polyurethanes. Ind Eng Chem Res 44:4504–4512
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Coats AW, Redfern JP (1965) Kinetic parameters from thermogravimetric data. II. J Polym Sci Part B Polym Lett 3:917–920
Doyle CD (1965) Series approximations to the equations of thermogravimetric data. Nature 207:290–291
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand 57:217–221
Akahira T, Sunose T (1971) Joint convection of four electrical institutes. Sci Technol 16:22–31
Flynn J, Wall L (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Pol Lett 4:323–328
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Sperling GR (1954) Hydrogen bonding in phenolic resin intermediates. J Am Chem Soc 76:1190–1193
Heal GR (2002) Thermogravimetry and derivative Thermogravimetry. In: Haines PJ (ed) Principles of thermal analysis and calorimetry. Royal Society of Chemistry, Cambridge, pp 10–54
Papadopoulou E, Chrissafis K (2011) Thermal study of phenol–formaldehyde resin modified with cashew nut shell liquid. Thermochim Acta 512:105–109
Idris SS, Rahman NA, Ismail K, Alias AB, Rashid ZA, Aris MJ (2010) Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour Technol 101:4584–4592
Lapuerta M, Hernández JJ, Rodrĺguez J (2004) Kinetics of devolatilisation of forestry wastes from thermogravimetric analysis. Biomass Bioenergy 27:385–391
Hamid R, Mostafa R, Faezeh M (2014) The non-isothermal degradation kinetics of St-MMA copolymers. Polym Degrad Stab 99:240–248
Budrugeac P, Homentcovschi D, Segal E (2001) Critical considerations on the isoconversional methods III. On the evaluation of the activation energy from non-isothermal data. J Therm Anal Calorim 66:557–565
Acknowledgements
The authors gratefully acknowledge the University Grants Commission (UGC), India, for financial assistance [Grant No. F.No.39-804/2010 (SR)] and the Principal and Management, PSG College of Technology, Coimbatore, for providing the necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natarajan, M., Murugavel, S.C. Thermal stability and thermal degradation kinetics of bio-based epoxy resins derived from cardanol by thermogravimetric analysis. Polym. Bull. 74, 3319–3340 (2017). https://doi.org/10.1007/s00289-016-1885-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1885-y