Abstract
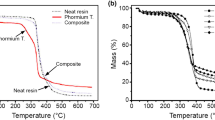
This paper presents the cure behavior and decomposition kinetics of epoxy resins incorporated with hydroxyl functionalized poly (ether ether ketone)s (PEEKTOH) and propargyl telechelic poly(ether ether ketone)s (PEEKPR). PEEKTOH and PEEKPR were blended with diglycidyl ether of bisphenol A (DGEBA) resin. The unmodified DGEBA without any PEEK modifier was also processed and evaluated for comparison. 4, 4′diamino diphenylsulfone was used as the curing agent for the blends. Non-isothermal cure kinetics was derived by differential scanning calorimetry, and the kinetic parameters including activation energy, pre-exponential factor and rate constant were determined. The cure initiation temperature was significantly influenced by the composition of the blend. Neat epoxy system without any modifier showed a cure initiation temperature in the range of 130–150 °C for different heating rates studied. PEEKTOH and PEEKPR modified epoxies exhibited cure initiation in the range of 118 to 145 °C and 121–149 °C, respectively, for different heating rates. Activation energies, Ea, at various extents of curing conversion were derived by Kissinger–Akahira–Sunose (KAS) method. The highest average activation energy range was exhibited by EP-Neat in the range of 77–80 kJ mol−1, whereas the EP-PEEKTOH showed Ea in the range of 66–70 kJ mol−1. Cured systems were subjected to thermal decomposition by thermogravimetric analysis. The activation energy for different extents of decomposition was studied by KAS method. The PEEKPR blended epoxy exhibited the highest values of activation energy for the entire range of decomposition, whereas the PEEKTOH system exhibited the lowest values.














Similar content being viewed by others
References
Mal H, Hul N, Wu C, Zhu Y, Cao Y, Chen QQ. Synthesis and research of epoxy resin toughening agent. Springerplus. 2016;806(5):1–10.
Sonnenfeld C, Jakani HM, Agogue R, Nunez P, Beauchene P. Thermoplastic/thermoset multilayer composites: A way to improve the impact damage tolerance of thermosetting resin matrix composites. Compos Struct. 2017;171(1):298–305.
Liu S, Chevali VS, Xu Z, Hui D, Wang H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos Part B-Eng. 2018;136(1):97–214.
Gibson G. Brydson’s Plastics Materials (Eighth Edition), Chapter 27 – Epoxy Resins. 2017; 773–97: https://doi.org/10.1016/B978-0-323-35824-8.00027-X
Morelle XP, Chevalier J, Bailly C, Pardoen T, Lani F. Mechanical characterization and modeling of the deformation and failure of the highly crosslinked RTM6 epoxy resin. Mech Time-Depend Mater. 2017;21:419–54.
Shin H, Kim B, GyuHan J, Lee MY, Park JK, Cho M. Fracture toughness enhancement of thermoplastic/epoxy blends by the plastic yield of toughening agents: A multiscale analysis. Compos Sci Technol. 2017;145:173–80.
Barbosa AQ, Silva LFM, Abenojar J, Figueiredo M, Ochsner A. Toughness of a brittle epoxy resin reinforced with micro cork particles: effect of size, amount and surface treatment. Compos Part B: Eng. 2017;114:299–310.
Wu X, Yang X, Yu R, Zhao XJ, Zhang Y, Huang Y. Highly crosslinked and uniform thermoset epoxy microspheres: Preparation and toughening study. Polymer. 2018;143:145–54.
Wang T, Huang P, Li YQ, Hu N, Fu SY. Epoxy nanocomposites significantly toughened by both poly(sulfone) and Grapheme oxide. Compos commun.2019; 14:55–60
Rosetti Y, Alcouffe P, Pascault JP, Gérard JF, Lortie F. Polyether Sulfone-Based Epoxy Toughening: From Micro- to Nano-Phase Separation via PES End-Chain Modification and Process Engineering. Materials (Basel). 2018; 11(10): https://doi.org/10.3390/ma11101960
Hong MH, Aravand MA, Falzon BG. Phase morphology and mechanical properties of polyetherimide modified epoxy resins: A comparative study. Polymer. 2019: 121640,
Zhao Q, Wang X, Hu Y. The application of highly soluble amine-terminated aromatic polyimides with pendent tert-butyl groups as a tougher for epoxy resin. Chin J Polym Sci. 2015;33:1359–72.
Hu JH, Yang B, Jin FL, Park SJ. Fracture toughness and surface morphology of polysulfone-modified epoxy resin. J Ind Eng Chem. 2015;25:9–11.
Francis B, Thomas S, Sadhana R, Thuaud N, Ramaswamy R. Diglycidyl Ether of Bisphenol-A Epoxy Resin Modified Using Poly(ether ether ketone) with Pendent tert-Butyl Groups. J Polym Sci Pol Phys. 2007;45:2481–96.
Francis B. Cure Kinetics of Epoxy/Thermoplastic Blends. In: Parameswaranpillai J, Hameed N, Pionteck J, Woo E, editors. Handbook of Epoxy Blends. Springer: Cham; 2017. p. 649–74.
Hua J, Shan J, Zhao J, Tong T. Isothermal curing kinetics of a flame retardant epoxy resin containing DOPO investigated by DSC and rheology. Thermochim Acta. 2016;632:56–63.
Granado L, Kempa S, Bremmert S, Gregoriades LJ, Brüning F, Anglaret E, Frety N. Isothermal DSC Study of the Curing Kinetics of an Epoxy/Silica Composite for Microelectronics. J Microelectron Electron Packag. 2017;14(2):45–50.
Luo X, Yu X, MA Y, Naito K, Zhang Q. Preparation and cure kinetics of epoxy with nanodiamond modified with liquid crystalline epoxy. Thermochim Acta. 2018; 663:1–8.
Liang JZ, Wang JZ, Tsui GCP, Tang CY. Thermal decomposition kinetics of polypropylene composites filled with rapheme nanoplatelets. Polymer Test. 2015;48:97–103.
Paluvai NR, Mohanty S, Nayak SK. Studies on thermal degradation and flame-retardant behavior of the sisal fiber reinforced unsaturated polyester toughened epoxy nanocomposites. J Appl Polym Sci. 2015;132(4):42068.
Katti P, Bose S, Kumar S. Tailored interface resulting in improvement in mechanical properties of epoxy composites containing poly (ether ether ketone) grafted multiwall carbon nanotubes. Polymer. 2016;102:43–53.
Augustine D, Vijayalakshmi KP, Sadhana R, Mathew D, Nair CPR. Hydroxyl terminated PEEK-toughened epoxyeaminonovolacphthalonitrile blends- Synthesis, cure studies and adhesive properties. Polymer. 2014;55(23):6006–16.
Bennett GS, Farris RJ, Thompson SA. Amine-terminated poly (aryl ether ketone)-epoxy/amine resin systems as tough high-performance materials. Polymer. 1991;32(9):1633–41.
Francis B, Ramaswam R, Rao VL, Thomas S. Toughening of Diglycidyl Ether of Bisphenol-A Epoxy Resin Using Poly (Ether Ether Ketone) with Pendent Ditert-Butyl Groups”. Int J Polym Mater Po. 2006;55(9):681–702.
Lee JS, Ko NY, Kwak NH, Ying WB, Lee B. Toughening of semi-IPN structured epoxy using a new PEEK-type polymer via in situ azide–alkyne click polymerization. J Appl Polym Sci. 2019;136:48178.
Luo Y, Zhang M, Dang G, Li Y, An X, Chen C, Yi X. Toughening of epoxy resin by poly (ether ether ketone) with pendant fluorocarbon groups. J Appl Polym Sci. 2011;122(3):1758–65.
Song X, Zheng S, Huang J, Zhu P, Guo Q. Miscibility and mechanical properties of tetrafunctional epoxy resin/phenolphthalein poly(ether ether ketone) blends. J Appl Polym Sci. 2000;79(4):598–607.
Francis B, Poel GV, Posada F, Groeninck G, Rao VL, Ramaswamy R, Thomas S. Cure kinetics and morphology of blends of epoxy resin with poly (ether ether ketone) containing pendant tertiary butyl groups. Polymer. 2003;44:3687–99.
Leena K, Mathew D, Robert TM. Poly(ether ether ketone)-bischromenes: Synthesis, characterization, and influence on thermal, mechanical, and thermo mechanical properties of epoxy resin. Polym Adv Technol. 2019;30(4):1061–71.
Sun Z, Xu L, Chen Z, Yuhao Wang Y, Tusiime R, Cheng C, Zhou S, Liu Y, Yu M, Zhang H. Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone. Polymers. 2019;11(3):461.
Varzeghani HN, Amraei IA, Mousavi SR. Dynamic Cure Kinetics and Physical-Mechanical Properties of PEG/Nanosilica/Epoxy Composites Hindawi International Journal of Polymer Science. 2020; Article ID 7908343. https://doi.org/10.1155/2020/7908343.
Shechter L, Wynstra J, Kurkjy RP. Glycidyl Ether Reactions with Amines. Ind Eng Chem. 1956;48(1):94–7.
Smith IT. The mechanism of the crosslinking of epoxide resins by amines. Polymer. 1961;2:95–108.
Wu SJ, Lin TK, Shyu SS. Cure Behavior, Morphology, and Mechanical Properties of the Melt Blends of Epoxy with Polyphenylene Oxide. J Appl Polym Sci. 2000;75:26–34.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Flynn J H, Wall LA. General Treatment of the Thermogravimetry of Polymers. J Res Natl Bur Stand. Sect. A 1966; 70: 487−523.
Ozawa T. Thermal Analysis− Review and Prospect. Thermochim Acta. 2000;355:35–42.
Fan M, Li X. Zhang, J. and Cheng, J. Curing kinetics and shape-memory behavior of an intrinsically toughened epoxy resin system. J Therm Anal Calorim. 2015; 119(1):537–546.
Chen M, Peng S, Zhao M, Liu Y, Liu C. The curing and degradation kinetics of modified epoxy–SiO2 composite. J Therm Anal Calorim. 2017;130:2123–31.
Vincent L, Mija A, Sbirrazzuoli N. Liquid crystalline and isotropic epoxy thermosets: mechanism and kinetics of non-isothermal degradation. Polymer Degrad Stabil. 2007;92(11):2051–7.
Pistor V, Ornaghi FG, Ornaghi HL Jr, Zattera AJ. Degradation kinetic of epoxy nanocomposites containing different percentage of epoxycyclohexyl—POSS. Polym Compos. 2012;33:1224–32.
Soares BG, Pistor C, Mauler BS. Influence of Different Percentages of N-Phenylaminopropyl—POSS on the Degradation Kinetic of Epoxy Resin. Polym Compos. 2012;33(12):1437–44.
Acknowledgements
The authors thank Director, VSSC, for granting permission to publish this work. They also thank Analytical and Spectroscopy Division, Vikram Sarabhai Space Centre (ISRO), for the support in characterization and testing of the materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karthikeyan, L., Desakumaran, D., Mol, P.B.S. et al. Non-isothermal cure and decomposition kinetics of hydroxyl and propargyl functional poly (ether ether ketone): epoxy resins. J Therm Anal Calorim 147, 6793–6805 (2022). https://doi.org/10.1007/s10973-021-11007-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11007-7