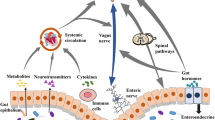
Abstract
The relationship between gut microbiota and pain, such as visceral pain, headaches (migraine), itching, prosthetic joint infection (PJI), chronic abdominal pain (CAP), joint pain, etc., has received increasing attention. Several parts of the evidence suggest that microbiota is one of the most important pain modulators and they can regulate pain in the central and peripheral nervous systems. Any alteration in microbiota by diet or antibiotics mediation may characterize a novel therapeutic strategy for pain management. The present study includes the most up-to-date and influential scientific findings on the association of microbiota with pain, despite the fact that the underlying mechanism is not identified in most cases. According to recent research, identifying the molecular mechanisms of the microbiota-pain pathway can have a unique perspective in treating many diseases, even though there is a long way to reach the ideal point. This study will stress the influence of microbiota on the common diseases that can stimulate the pain with a focus on underlying mechanisms.





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Arora HC, Eng C, Shoskes DA (2017) Gut microbiome and chronic prostatitis/chronic pelvic pain syndrome. Ann Transl Med 5(2):30–30. https://doi.org/10.21037/atm.2016.12.32
Blake KJ et al (2018) Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat Commun 9(1):1–15. https://doi.org/10.1038/s41467-017-02448-6
Russo R et al (2018) Gut–brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 25(32):3930–3952. https://doi.org/10.2174/0929867324666170216113756
Gomaa EZ (2020) Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113(12):2019–2040. https://doi.org/10.1007/s10482-020-01474-7
Guo R et al (2019) Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 123(5):637–654. https://doi.org/10.1016/j.bja.2019.07.026
Madanraj AS et al (2019) Interventions to chronic prostatitis/chronic pelvic pain syndrome treatment. Where are we standing and what’s next? Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2019.172429
Zheng D, Liwinski T, Elinav E (2020) Interaction between microbiota and immunity in health and disease. Cell Res 30(6):492–506. https://doi.org/10.1038/s41422-020-0332-7
Vendrik KE et al (2020) Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol 10:98. https://doi.org/10.3389/fcimb.2020.00098
Shin A et al (2019) The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol 17(2):256–274. https://doi.org/10.1016/j.cgh.2018.08.054
Freria C et al (2016) Impairment of toll-like receptors 2 and 4 leads to compensatory mechanisms after sciatic nerve axotomy. J Neuroinflamm 13(1):118. https://doi.org/10.1186/s12974-016-0579-6
Ren Y et al (2021) Transcriptome-wide identification and characterization of toll-like receptors response to Vibrio anguillarum infection in Manila clam (Ruditapes philippinarum). Fish Shellfish Immunol 111:49–58. https://doi.org/10.1016/j.fsi.2021.01.007
Siegel SJ, Rakoff-Nahoum S (2019) Innate immune pattern recognition and the development of intestinal cancer. In: Robertson ES (ed) Microbiome and cancer. Springer, Cham, pp 299–316. https://doi.org/10.1007/978-3-030-04155-7_14
Patin EC, Thompson A, Orr SJ (2019) Pattern recognition receptors in fungal immunity. In: Davey J (ed) Seminars in cell & developmental biology. Elsevier, Amsterdam. https://doi.org/10.1016/j.semcdb.2018.03.003
Fan VS et al (2016) COPD disease severity and innate immune response to pathogen-associated molecular patterns. Int J Chron Obstruct Pulmon Dis 11:467. https://doi.org/10.2147/COPD.S94410
Dubový P et al (2021) Toll-like receptor 9-mediated neuronal innate immune reaction is associated with initiating a pro-regenerative state in neurons of the dorsal root ganglia non-associated with sciatic nerve lesion. Int J Mol Sci 22(14):7446. https://doi.org/10.3390/ijms22147446
Thakur KK et al (2017) Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol Res 115:224–232. https://doi.org/10.1016/j.phrs.2016.11.019
Li L et al (2021) Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav Immun 91:740–755. https://doi.org/10.1016/j.bbi.2020.10.007
Spiljar M, Merkler D, Trajkovski M (2017) The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, mucosal barrier, and SCFAs. Front Immunol 8:1353. https://doi.org/10.3389/fimmu.2017.01353
Chatoo M et al (2018) Involvement of corticotropin-releasing factor and receptors in immune cells in irritable bowel syndrome. Front Endocrinol 9:21. https://doi.org/10.3389/fendo.2018.00021
Wei L et al (2021) Gut microbiota dysbiosis in functional gastrointestinal disorders: underpinning the symptoms and pathophysiology. JGH Open 5(9):976–987. https://doi.org/10.1002/jgh3.12528
Li S et al (2020) The role of bacteria and its derived metabolites in chronic pain and depression: recent findings and research progress. Int J Neuropsychopharmacol 23(1):26–41. https://doi.org/10.1093/ijnp/pyz061
Wrba L et al (2022) Adipose tissue: a neglected organ in the response to severe trauma? Cell Mol Life Sci 79(4):1–12. https://doi.org/10.1007/s00018-022-04234-0
Borrelli A et al (2018) Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol 15:467–479. https://doi.org/10.1016/j.redox.2018.01.009
O’Mahony SM, Dinan TG, Cryan JF (2017) The gut microbiota as a key regulator of visceral pain. Pain 158:19–28. https://doi.org/10.1097/j.pain.0000000000000779
Rea K et al (2017) The role of the gastrointestinal microbiota in visceral pain. Gastrointestinal pharmacology. Springer, New York, pp 269–287
Goodoory VC et al (2022) Direct healthcare costs of Rome IV or Rome III-defined irritable bowel syndrome in the United Kingdom. Aliment Pharmacol Ther 56(1):110–120
Staudacher HM, Whelan K (2016) Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: probiotics, prebiotics and the low FODMAP diet. Proc Nutr Soc 75(3):306–318. https://doi.org/10.1017/S0029665116000021
O’Mahony SM, Dinan TG, Cryan JF (2017) The gut microbiota as a key regulator of visceral pain. Pain 158:S19–S28. https://doi.org/10.1097/j.pain.0000000000000779
Pusceddu MM, Gareau MG (2018) Visceral pain: gut microbiota, a new hope? J Biomed Sci 25(1):73. https://doi.org/10.1186/s12929-018-0476-7
Fukui H, Xu X, Miwa H (2018) Role of gut microbiota–gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J Neurogastroenterol Motil 24(3):367. https://doi.org/10.5056/jnm18071
Mars RA, Frith M, Kashyap PC (2021) Functional gastrointestinal disorders and the microbiome-what is the best strategy for moving microbiome-based therapies for functional gastrointestinal disorders into the clinic? Gastroenterology 160(2):538–555. https://doi.org/10.1053/j.gastro.2020.10.058
Wood D (2013) Taming the irritable bowel. Current Pharm Design 19(1):142–156
Jeffery IB et al (2020) Microbiome alterations in IBS. Gut. https://doi.org/10.1136/gutjnl-2020-320919
Reynolds C et al (2018) Fecal microbiome patterns in IBS, IBD, and celiac disease versus healthy controls. Am J Gastroenterol 113:272–273. https://doi.org/10.14309/00000434-201810001-00472
Chong PP et al (2019) The microbiome and irritable bowel syndrome-a review on the pathophysiology, current research and future therapy. Front Microbiol 10:1136. https://doi.org/10.3389/fmicb.2019.01136
Zhuang X et al (2018) Fecal microbiota alterations associated with diarrhea-predominant irritable bowel syndrome. Front Microbiol 9:1600. https://doi.org/10.3389/fmicb.2018.01600
Kundu P et al (2017) Our gut microbiome: the evolving inner self. Cell 171(7):1481–1493. https://doi.org/10.1016/j.cell.2017.11.024
Saffouri GB et al (2019) Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun 10(1):1–11. https://doi.org/10.1038/s41467-019-09964-7
Kaplan H, Hutkins RW (2000) Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol 66(6):2682–2684. https://doi.org/10.1128/AEM.66.6.2682-2684.2000
Rivière A et al (2018) Complementary mechanisms for degradation of inulin-type fructans and arabinoxylan oligosaccharides among bifidobacterial strains suggest bacterial cooperation. Appl Environ Microbiol 84(9):e02893-e2917. https://doi.org/10.1128/AEM.02893-17
Silk D et al (2009) Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 29(5):508–518. https://doi.org/10.1111/j.1365-2036.2008.03911.x
Nielsen TK et al (2019) A porcine model for urinary tract infection. Front Microbiol 10:2564. https://doi.org/10.3389/fmicb.2019.02564
Berrondo C et al (2017) PD05-10 antibiotic prophylaxis prior to urinary catheter removal after radical prostatectomy does not prevent urinary tract infections: a randomized controlled clinical trial. J Urol 197(4S):e120–e120. https://doi.org/10.1016/j.juro.2017.02.359
Rosen JM, Klumpp DJ (2014) Mechanisms of pain from urinary tract infection. Int J Urol 21(S1):26–32. https://doi.org/10.1111/iju.12309
Du H-X et al Abnormal gut microbiota composition is associated with experimental autoimmune prostatitis-induced depressive-like behaviors in mice. Prostate. n/a(n/a)
Golombos DM et al (2018) The role of gut microbiome in the pathogenesis of prostate cancer: a prospective pilot study. Urology 111:122–128. https://doi.org/10.1016/j.urology.2017.08.039
Massari F et al (2019) The human microbiota and prostate cancer: friend or foe? Cancers 11(4):459. https://doi.org/10.3390/cancers11040459
Sfanos KS et al (2018) The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol 15(1):11–24. https://doi.org/10.1038/nrurol.2017.167
Porter CM et al (2018) The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis 21(3):345–354. https://doi.org/10.1038/s41391-018-0041-1
Krsmanovic A et al (2014) Psychosocial mechanisms of the pain and quality of life relationship for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Can Urol Assoc J 8(11–12):403. https://doi.org/10.5489/cuaj.2179
Shoskes DA, Nickel JC (2013) Classification and treatment of men with chronic prostatitis/chronic pelvic pain syndrome using the UPOINT system. World J Urol 31(4):755–760. https://doi.org/10.1007/s00345-013-1075-6
Shoskes DA et al (2016) Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J Urol 196(2):435–441. https://doi.org/10.1016/j.juro.2016.02.2959
Shoskes DA et al (2016) The urinary microbiome differs significantly between patients with chronic prostatitis/chronic pelvic pain syndrome and controls as well as between patients with different clinical phenotypes. Urology 92:26–32. https://doi.org/10.1016/j.urology.2016.02.043
Breser ML et al (2017) Immunological mechanisms underlying chronic pelvic pain and prostate inflammation in chronic pelvic pain syndrome. Front Immunol. https://doi.org/10.3389/fimmu.2017.00898
Shoskes DA et al (1999) Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology 54(6):960–963. https://doi.org/10.1016/S0090-4295(99)00358-1
Noghani MT, Namdar H (2019) Migraine associated with gastrointestinal disorders: a pathophysiological explanation. Med Hypotheses 125:90–93. https://doi.org/10.1016/j.mehy.2019.02.041
Rahmoune H, Boutrid N (2017) Migraine, celiac disease and intestinal microbiota. Pediatr Neurol Briefs 31(2):6. https://doi.org/10.15844/pedneurbriefs-31-2-3
Yu-Jie Dai M et al (2017) Potential beneficial effects of probiotics on human migraine headache: a literature review. Pain Phys 20:S251–S255. https://doi.org/10.36076/ppj.2017.E255
Tang Y et al (2020) Gut microbiota dysbiosis enhances migraine-like pain via TNFα upregulation. Mol Neurobiol 57(1):461–468. https://doi.org/10.1007/s12035-019-01721-7
Cámara-Lemarroy CR et al (2016) Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol 22(36):8149. https://doi.org/10.3748/wjg.v22.i36.8149
Talafi Noghani M, Namdar H (2019) Migraine associated with gastrointestinal disorders: A pathophysiological explanation. Med Hypotheses 125:90–93. https://doi.org/10.1016/j.mehy.2019.02.041
Firestein GS, McInnes IB (2017) Immunopathogenesis of rheumatoid arthritis. Immunity 46(2):183–196. https://doi.org/10.1016/j.immuni.2017.02.006
Horta-Baas G et al (2017) Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. J Immunol Res. https://doi.org/10.1155/2017/4835189
Xu X et al (2021) Gut–bone axis: a non-negligible contributor to periodontitis. Front Cell Infect Microbiol 11:1135
Boer CG et al (2019) Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun 10(1):1–9. https://doi.org/10.1038/s41467-019-12873-4
Bergot A-S, Giri R, Thomas R (2019) The microbiome and rheumatoid arthritis. Best Pract Res Clin Rheumatol 33(6):101497. https://doi.org/10.1016/j.berh.2020.101497
Clos-Garcia M et al (2019) Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 46:499–511. https://doi.org/10.1016/j.ebiom.2019.07.031
Qassem R, Watad A (2021) The role of infection and immunization in the induction of fibromyalgia. Fibromyalgia syndrome. Springer, New York, pp 267–272
Minerbi A, Fitzcharles M (2020) Gut microbiome: pertinence in fibromyalgia. Clin Exp Rheumatol 38(1):99
Erdrich S et al (2020) Determining the association between fibromyalgia, the gut microbiome and its biomarkers: a systematic review. BMC Musculoskelet Disord 21(1):1–12. https://doi.org/10.1186/s12891-020-03201-9
Minerbi A, Fitzcharles M-A (2020) Gut microbiome: pertinence in fibromyalgia. Clin Exp Rheumatol 38(123):S99–S104
Minerbi A et al (2019) Altered microbiome composition in individuals with fibromyalgia. Pain 160(11):2589–2602. https://doi.org/10.1097/j.pain.0000000000001640
Nickel JC et al (2018) The interstitial cystitis/bladder pain syndrome clinical picture: a perspective from patient life experience. Urol Pract 5(4):286–292. https://doi.org/10.1016/j.urpr.2017.06.005
Colemeadow J, Sahai A, Malde S (2020) Clinical management of bladder pain syndrome/interstitial cystitis: a review on current recommendations and emerging treatment options. Res Rep Urol 12:331. https://doi.org/10.2147/RRU.S238746
Thomas-White K et al (2018) Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun 9(1):1–7. https://doi.org/10.1038/s41467-018-03968-5
Wein AJ (2020) Re: A culture-independent analysis of the microbiota of female interstitial cystitis/bladder pain syndrome participants in the MAPP research network. J Urol 203(2):258–259. https://doi.org/10.1097/01.JU.0000614912.86465.ab
Grundy L, Caldwell A, Brierley SM (2018) Mechanisms underlying overactive bladder and interstitial cystitis/painful bladder syndrome. Front Neurosci 12:931. https://doi.org/10.3389/fnins.2018.00931
Contreras-Sanz A et al (2016) Altered urothelial ATP signaling in a major subset of human overactive bladder patients with pyuria. Am J Physiol-Renal Physiol 311(4):F805–F816. https://doi.org/10.1152/ajprenal.00339.2015
Nickel JC et al (2019) A culture-independent analysis of the microbiota of female interstitial cystitis/bladder pain syndrome participants in the MAPP research network. J Clin Med 8(3):415. https://doi.org/10.3390/jcm8030415
Curtiss N et al (2017) A case controlled study examining the bladder microbiome in women with overactive bladder (OAB) and healthy controls. Eur J Obstet Gynecol Reprod Biol 214:31–35. https://doi.org/10.1016/j.ejogrb.2017.04.040
Bajic P, Wolfe AJ, Gupta GN (2019) The urinary microbiome: implications in bladder cancer pathogenesis and therapeutics. Urology 126:10–15. https://doi.org/10.1016/j.urology.2018.12.034
Abernethy MG et al (2017) Urinary microbiome and cytokine levels in women with interstitial cystitis. Obstet Gynecol 129(3):500–506. https://doi.org/10.1097/AOG.0000000000001892
Guida F et al (2020) Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immunol 85:128–141. https://doi.org/10.1016/j.bbi.2019.04.006
Burmistr I (2018) Theories of pain, up to Descartes and after neuromatrix: what role do they have to develop future paradigms? Pain Med 3(1):6–12. https://doi.org/10.31636/pmjua.v3i1.81
Singhal M, Arora V, Im H-J (2020) Gastrointestinal disorders-induced pain. Gene Rep 18:100580. https://doi.org/10.1016/j.genrep.2019.100580
Eicher TP, Mohajeri MH (2022) Overlapping mechanisms of action of brain-active bacteria and bacterial metabolites in the pathogenesis of common brain diseases. Nutrients 14(13):2661. https://doi.org/10.3390/nu14132661
Liang Y et al (2021) Gut microbial metabolites in Parkinson’s disease: implications of mitochondrial dysfunction in the pathogenesis and treatment. Mol Neurobiol 58(8):3745–3758. https://doi.org/10.1007/s12035-021-02375-0
Favazzo LJ, Hendesi H, Villani DA, Soniwala S, Dar Q-A, Schott EM et al (2020) The gut microbiome–joint connection: implications in osteoarthritis. Curr Opin Rheumatol 32(1):92–101. https://doi.org/10.1097/bor.0000000000000681
Rea K, O’Mahony SM, Dinan TG, Cryan JF (2017) The role of the gastrointestinal microbiota in visceral pain. In: Greenwood-Van Meerveld B (ed) Gastrointestinal pharmacology. Springer, New York, pp 269–287
O’Mahony SM, Dinan TG, Cryan JF (2017) The gut microbiota as a key regulator of visceral pain. Pain. https://doi.org/10.1097/j.pain.0000000000000779
van Hemert S, Breedveld AC, Rovers JMP, Vermeiden JPW, Witteman BJM, Smits MG et al (2014) Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol. https://doi.org/10.3389/fneur.2014.00241
Simrén M (2018) Manipulating the gut microbiome as a treatment strategy for functional gastrointestinal disorders. Gastroenterology 155(4):960–962. https://doi.org/10.1053/j.gastro.2018.09.008
Malt EA, Olafsson S, Ursin H (2004) Fibromyalgia a manifestation of helicobacter pylori infection? Scand J Rheumatol 33(2):131. https://doi.org/10.1080/03009740410006826-1468
Minerbi A, Gonzalez E, Brereton NJB, Anjarkouchian A, Dewar K, Fitzcharles M-A et al (2019) Altered microbiome composition in individuals with fibromyalgia. Pain 160(11):2589–2602. https://doi.org/10.1097/j.pain.0000000000001640
Peña-Vélez R, Toro-Monjaraz E, Avelar-Rodríguez D, Ignorosa-Arellano K, Zárate-Mondragón F, Cervantes-Bustamante R et al (2019) Small intestinal bacterial overgrowth: could it be associated with chronic abdominal pain in children with allergic diseases? REV ESP ENFERM DIG 111(12):927–930. https://doi.org/10.17235/reed.2019.6321/2019
Sekharan C, Kumar D, Kumari K, Joachim C (2017) Determination of prevalence of urinary tract infection among the pregnant women with lower abdominal pain. UK J Pharm Biosci 5(2):50–55. https://doi.org/10.20510/ukjpb/5/i2/155996
Schmulson M, Bielsa MV, Carmona-Sánchez R, Hernández A, López-Colombo A, Vidal YL et al (2014) Microbiota, gastrointestinal infections, low-grade inflammation, and antibiotic therapy in irritable bowel syndrome (IBS): an evidence-based review. Revista de Gastroenterología de México (English Edition) 79(2):96–134. https://doi.org/10.1016/j.rgmxen.2014.01.001
Boff D, Oliveira VLS, Queiroz Junior CM, Silva TA, Allegretti M, Verri WA Jr et al (2018) CXCR2 is critical for bacterial control and development of joint damage and pain in Staphylococcus aureus-induced septic arthritis in mouse. Eur J Immunol 48(3):454–463. https://doi.org/10.1002/eji.201747198
Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P et al (2019) Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun 10(1):4881. https://doi.org/10.1038/s41467-019-12873-4
Rosen JM, Klumpp DJ (2014) Mechanisms of pain from urinary tract infection. Int J Urol 21:26–32. https://doi.org/10.1111/iju.12309
Nickel J (2016) Inflammatory and pain conditions of the male genitourinary tract: prostatitis and related pain conditions, orchitis, and epididymitis, 11th edn. Elsevier, Philadelphia
Salehi B, Butnariu M, Corneanu M, Sarac I, Vlaisavljevic S, Kitic D et al (2019) Chronic pelvic pain syndrome: highlighting medicinal plants toward biomolecules discovery for upcoming drugs formulation. Phytother Res. https://doi.org/10.1002/ptr.6576
Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R et al (2018) Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev 123:3–17. https://doi.org/10.1016/j.addr.2017.09.018
Kundu P, Blacher E, Elinav E, Pettersson S (2017) Our gut microbiome: the evolving inner self. Cell 171(7):1481–1493. https://doi.org/10.1016/j.cell.2017.11.024
Fukui H, Xu X, Miwa H (2018) Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J Neurogastroenterol Motil 24(3):367. https://doi.org/10.5056/jnm18071
Barbara G, Feinle-Bisset C, Ghoshal UC, Santos J, Vanner SJ, Vergnolle N et al (2016) The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 150(6):1305–18.e8. https://doi.org/10.1053/j.gastro.2016.02.028
Nickel JC, Stephens-Shields AJ, Landis JR, Mullins C, van Bokhoven A, Lucia MS et al (2019) A culture-independent analysis of the microbiota of female interstitial cystitis/bladder pain syndrome participants in the MAPP research network. J Clin Med 8(3):415. https://doi.org/10.3390/jcm8030415
Southgate EL, He RL, Gao JL, Murphy PM, Nanamori M, Ye RD (2008) Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J Immunol 181(2):1429–1437. https://doi.org/10.4049/jimmunol.181.2.1429
Luo Z, Wang H, Fang S, Li L, Li X, Shi J et al (2019) Annexin-1 mimetic peptide Ac2–26 suppresses inflammatory mediators in LPS-induced astrocytes and ameliorates pain hypersensitivity in a rat model of inflammatory pain. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-019-00755-8
Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J et al (2013) Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501(7465):52–57. https://doi.org/10.1038/nature12479
Rosen JM, Yaggie RE, Woida PJ, Miller RJ, Schaeffer AJ, Klumpp DJ (2018) TRPV1 and the MCP-1/CCR2 axis modulate post-UTI chronic pain. Sci Rep 8(1):7188. https://doi.org/10.1038/s41598-018-24056-0
Blake KJ, Baral P, Voisin T, Lubkin A, Pinho-Ribeiro FA, Adams KL et al (2018) Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat Commun 9(1):37. https://doi.org/10.1038/s41467-017-02448-6
Acknowledgements
The authors would like to thank the Aging Research Institute and the Physical Medicine Rehabilitation Research Center of Tabriz University of Medical Sciences.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AM; Writing and editing original draft, NA: Writing and editing original draft, GN: Writing original draft and tables drawing, MsK: Writing and editing original draft, TH: Writing and editing original draft, MEs-a: Writing and editing original draft.
Corresponding authors
Ethics declarations
Conflict of interest
There is no declared conflict of interest.
Ethical Approval
The paper does not contain any study on human participants or animals.
Consent to Participate
Not applicable.
Consent for Publication
All authors read and approved the content of the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alizadeh, N., Naderi, G., Kahrizi, M.s. et al. Microbiota-Pain Association; Recent Discoveries and Research Progress. Curr Microbiol 80, 29 (2023). https://doi.org/10.1007/s00284-022-03124-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03124-9