Abstract
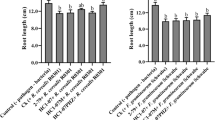
Tomato is one of the most economically attractive vegetable crops due to its high yields. Diseases cause significant losses in tomato production worldwide. We carried out Polymerase Chain Reaction studies to detect the presence of genes encoding antifungal compounds in the DNA of Pseudomonas putida strain PCI2. We also used liquid chromatography-electrospray tandem mass spectrometry to detect and quantify the production of compounds that increase the resistance of plants to diseases from culture supernatants of PCI2. In addition, we investigated the presence of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase in PCI2. Finally, PCI2 was used for inoculation of tomato seeds to study its potential biocontrol activity against Fusarium oxysporum MR193. The obtained results showed that no fragments for the encoding genes of hydrogen cyanide, pyoluteorin, 2,4-diacetylphloroglucinol, pyrrolnitrin, or phenazine-1-carboxylic acid were amplified from the DNA of PCI2. On the other hand, PCI2 produced salicylic acid and jasmonic acid in Luria–Bertani medium and grew in a culture medium containing ACC as the sole nitrogen source. We observed a reduction in disease incidence from 53.33 % in the pathogen control to 30 % in tomato plants pre-inoculated with PCI2 as well as increases in shoot and root dry weights in inoculated plants, as compared to the pathogenicity control. This study suggests that inoculation of tomato seeds with P. putida PCI2 increases the resistance of plants to root rot caused by F. oxysporum and that PCI2 produces compounds that may be involved at different levels in increasing such resistance. Thus, PCI2 could represent a non-contaminating management strategy potentially applicable in vegetable crops such as tomato.



Similar content being viewed by others
References
An CF, Mou ZL (2011) Salicylic acid and its function in plant immunity. J Integr Plant Biol 53:412–428
Anjaiah V (2004) Biological control mechanisms of fluorescent Pseudomonas species involved in control of root diseases of vegetables/fruits. In: Mukerji KG (ed) Fruit and vegetables diseases. Springer, Netherlands, pp 453–500
Bakker PAHM, Ran LX, Pieterse CMJ, Van Loon LC (2003) Understanding the involvement of induced systemic resistance in rhizobacteria-mediated biocontrol of plant diseases. Can J Plant Pathol 25:5–9
Bakker PAHM, Pieterse CMJ, van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 97:239–243
Barnawal D, Bharti N, Maji D, Singh Chanotiya C, Kalra A (2012) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol Biochem 58:227–235
Baysal Ö, Çalışkan M, Yeşilova Ö (2008) An inhibitory effect of a new Bacillus subtilis strain (EU07) against Fusarium oxysporum f. sp. radicis-lycopersici. Physiol Mol Plant Pathol 73:25–32
Benhamou N, Lafontaine PJ, Nicole M (1994) Induction of systemic resistance to fusarium crown and root rot in tomato plants by seed treatment with chitosan. Phytopathology 84:1432–1444
Bi HH, Zeng RS, Su LM, An M, Luo SM (2007) Rice allelopathy induced by methyl jasmonate and methyl salicylate. J Chem Ecol 33:1089–1103
Brayford D (1996) IMI descriptions of fungi and bacteria set 127. Mycopathologia 133:35–63
Cavalca L, Zanchi R, Corsini A, Colombo M, Romagnoli C, Canzi E, Andreoni V (2010) Arsenic-resistant bacteria associated with roots of the wild Cirsium arvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst Appl Microbiol 33:154–164
Datnoff LE, Nemec S, Pernezny K (1995) Biological control of Fusarium crown and root rot of tomato in Florida using Trichoderma harzianum and Glomus intraradices. Biol Control 5:427–431
Etesami H, Hosseini HM, Alikhani HA, Mohammadi L (2014) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (iaa) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul 33:654–670
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Khalil S, Alsanius BW (2010) Evaluation of biocontrol agents for managing root diseases on hydroponically grown tomato. J Plant Dis Prot 117:214–219
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, Pennsylvania
Ozbay N, Newman SE (2004) Fusarium crown and root rot of tomato and control methods. Plant Pathol J 3:9–18
Pastor NA, Reynoso MM, Tonelli ML, Masciarelli O, Rosas SB, Rovera M (2010) Potential biological control Pseudomonas sp. PCI2 against damping-off of tomato caused by Sclerotium rolfsii. J Plant Pathol 92:737–745
Pastor NA, Rosas SB, Andrés J, Niederhauser MC, Rovera M (2013) Biocontrol of fungal diseases of tomato: contribution of Pseudomonas sp. PCI2. In: Higashide T (ed) Tomatoes: cultivation, varieties and nutrition. Nova Science Publishers, New York, pp 339–351
Pastor N, Rosas S, Luna V, Rovera M (2014) Inoculation with Pseudomonas putida PCI2, a phosphate solubilizing rhizobacterium, stimulates the growth of tomato plants. Symbiosis 62:157–167
Raaijmakers JM, Weller DM, Thomashow LS (1997) Frequency of Antibiotic-Producing Pseudomonas spp. in Natural Environments. Appl Environ Microbiol 63:881–887
Ramette A, Frapolli M, Défago G, Moënne-Loccoz Y (2003) Phylogeny of HCN synthase-encoding hcnBC genes in biocontrol fluorescent pseudomonads and its relationship with host plant species and HCN synthesis ability. Mol Plant-Microbe Interact 16:525–535
Ran LX, Li ZN, Wu GJ, van Loon LC, Bakker PAHM (2005) Induction of systemic resistance against bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. Eur J Plant Pathol 113:59–70
Ran LX, van Loon LC, Bakker PA (2005) No role for bacterially produced salicylic acid in rhizobacterial induction of systemic resistance in Arabidopsis. Phytopathology 95:1349–1355
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. CSHL Press, New York
Shakir MA, Bano A, Arshad M (2012) Rhizosphere bacteria containing ACC-deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ 31:108–112
Siddikee A, Chauhan PS, Sa T (2012) Regulation of ethylene biosynthesis under salt stress in red pepper (Capsicum annuum L.) by 1-Aminocyclopropane-1-Carboxylic acid (ACC) deaminase-producing halotolerant bacteria. J Plant Growth Regul 31:265–272
Souza JT, Raaijmakers JM (2003) Polymorphisms within the prnD and pltC genes from pyrrolnitrin and pyoluteorin-producing Pseudomonas and Burkholderia spp. FEMS Microbiol Ecol 43:21–34
Svercel M, Duffy B, Défago G (2007) PCR amplification of hydrogen cyanide biosynthetic locus hcnAB in Pseudomonas spp. J Microbiol Methods 70:209–213
van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
van Loon LC, Bakker PA, Pieterse CM (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
van Wees SCM, de Swart EAM, van Pelt JA, van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci 97:8711–8716
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Acknowledgments
This work was supported by grants from Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto (Córdoba, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (Secretaría de Ciencia y Técnica de la Nación), and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pastor, N., Masciarelli, O., Fischer, S. et al. Potential of Pseudomonas putida PCI2 for the Protection of Tomato Plants Against Fungal Pathogens. Curr Microbiol 73, 346–353 (2016). https://doi.org/10.1007/s00284-016-1068-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1068-y