Abstract
Purpose
Acid ceramidase (AC) occupies an important place in the control of cancer cell proliferation. We tested the influence of AC inhibition on the effects of PSC 833, a P-glycoprotein antagonist with potent ceramide-generating capacity, to determine whether AC could be a therapeutic target in pancreatic cancer.
Methods
Ceramide metabolism was followed using 3H-palmitate, and molecular species were determined by mass spectroscopy. Apoptosis was measured by DNA fragmentation, autophagy by acridine orange staining, and cell cycle was assessed by flow cytometry and RB phosphorylation. AC was measured in intact cells using fluorescent substrate.
Results
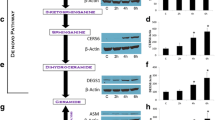
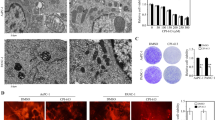
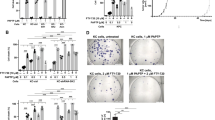
Exposure of human PANC-1 or MIA-PaCa-2 cells to PSC 833 promoted increases in de novo (dihydro)ceramides, (dihydro)glucosylceramides, and (dihydro)sphingomyelins, demarking ceramide generation and robust metabolism. Despite the multifold increases in (dihydro)ceramide levels, cells were refractory to PSC 833. However, PSC 833 produced a dose-dependent decrease in DNA synthesis and dose- and time-dependent decreases in RB phosphorylation, consistent with cell cycle arrest as demonstrated at G1. Cytostatic effects of PSC 833 were converted to cytotoxic end-point by acid ceramidase inhibition. Cytotoxicity was accompanied by formation of acridine orange-stained acidic vesicles and an increase in LC3 expression, indicative of autophagic response. Cell death was not reversed by preexposure to myriocin, which blocks PSC 833-induced ceramide generation.
Conclusion
Although the role of ceramide in end-point cytotoxicity is unclear, our results suggest that acid ceramidase is a viable target in pancreatic cancer. We propose that AC inhibition will be effective in concert with other anticancer therapies.






Similar content being viewed by others
Abbreviations
- DHCer:
-
Dihydroceramide
- DHGlcCer:
-
Dihydroglucosylceramide
- DHSM:
-
Dihydrosphingomyelin
- dThd:
-
[3H]thymidine
- FBS:
-
Fetal bovine serum
- GlcCer:
-
Glucosylceramide
- LC MS/MS:
-
Liquid chromatography/electrospray tandem mass spectrometry
- LSC:
-
Liquid scintillation counting
- MDR-1:
-
Multidrug resistant-1
- MTS:
-
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- P-gp:
-
P-glycoprotein
- PI:
-
Propidium iodide
- S1-P:
-
Sphingosine 1-phosphate
- SM:
-
Sphingomyelin
- SPT:
-
Serine palmitoyltransferase
References
Abe A, Radin NS, Shayman JA, Wotring LL, Zipkin RE, Sivakumar R, Ruggieri JM, Carson KG, Ganem B (1995) Structural and stereochemical studies of potent inhibitors of glucosylceramide synthase and tumor cell growth. J Lipid Res 36:611–621
Bedia C, Canals D, Matabosch X, Harrak Y, Casas J, Llebaria A, Delgado A, Fabrias G (2008) Cytotoxicity and acid ceramidase inhibitory activity of 2-substituted aminoethanol amides. Chem Phys Lipids 156:33–40
Bedia C, Casas J, Garcia V, Levade T, Fabrias G (2007) Synthesis of a novel ceramide analogue and its use in a high-throughput fluorogenic assay for ceramidases. ChemBioChem 8:642–648
Bedia C, Levade T, Codogno P (2011) Regulation of autophagy by sphingolipids. Anticancer Agents Med Chem 11:844–853
Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC (2005) Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene 24:178–187
Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C, Hannun YA (1996) (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J Biol Chem 271:12646–12654
Bleicher RJ, Cabot MC (2002) Glucosylceramide synthase and apoptosis. Biochim Biophys Acta 1585:172–178
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Cabot MC, Han TY, Giuliano AE (1998) The multidrug resistance modulator SDZ PSC 833 is a potent activator of cellular ceramide formation. FEBS Lett 431:185–188
Canals D, Perry DM, Jenkins RW, Hannun YA (2011) Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol 163:694–712
Eckstein LA, Van Quill KR, Bui SK, Uusitalo MS, O’Brien JM (2005) Cyclosporin a inhibits calcineurin/nuclear factor of activated T-cells signaling and induces apoptosis in retinoblastoma cells. Invest Ophthalmol Vis Sci 46:782–790
Fabrias G, Bedia C, Casas J, Abad JL, Delgado A (2011) Ceramidases in hematological malignancies: senseless or neglected target? Anticancer Agents Med Chem 11:830–843
Flowers M, Fabrias G, Delgado A, Casas J, Abad JL, Cabot MC (2012) C6-Ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res Treat 133:447–458
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319
Gatt S (1963) Enzymic hydrolysis and synthesis of ceramides. J Biol Chem 238:3131–3133
Gouaze-Andersson V, Cabot MC (2011) Sphingolipid metabolism and drug resistance in hematological malignancies. Anticancer Agents Med Chem 11:891–903
Gouaze-Andersson V, Flowers M, Karimi R, Fabrias G, Delgado A, Casas J, Cabot MC (2011) Inhibition of acid ceramidase by a 2-substituted aminoethanol amide synergistically sensitizes prostate cancer cells to N-(4-hydroxyphenyl) retinamide. Prostate 71:1064–1073
Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, Delisle MB, Cuvillier O, Susini C, Bousquet C (2009) Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther 8:809–820
Juul N, Szallasi Z, Eklund AC, Li Q, Burrell RA, Gerlinger M, Valero V, Andreopoulou E, Esteva FJ, Symmans WF, Desmedt C, Haibe-Kains B, Sotiriou C, Pusztai L, Swanton C (2010) Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: a retrospective analysis of five clinical trials. Lancet Oncol 11:358–365
Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, Keyomarsi K (2008) Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res 68:7966–7974
Lehne G, Sorensen DR, Tjonnfjord GE, Beiske C, Hagve TA, Rugstad HE, Clausen OP (2002) The cyclosporin PSC 833 increases survival and delays engraftment of human multidrug-resistant leukemia cells in xenotransplanted NOD-SCID mice. Leukemia 16:2388–2394
Leonard GD, Polgar O, Bates SE (2002) ABC transporters and inhibitors: new targets, new agents. Curr Opin Investig Drugs 3:1652–1659
Liu X, Cheng JC, Turner LS, Elojeimy S, Beckham TH, Bielawska A, Keane TE, Hannun YA, Norris JS (2009) Acid ceramidase upregulation in prostate cancer: role in tumor development and implications for therapy. Expert Opin Ther Targets 13:1449–1458
Liu X, Elojeimy S, Turner LS, Mahdy AE, Zeidan YH, Bielawska A, Bielawski J, Dong JY, El-Zawahry AM, Guo GW, Hannun YA, Holman DH, Rubinchik S, Szulc Z, Keane TE, Tavassoli M, Norris JS (2008) Acid ceramidase inhibition: a novel target for cancer therapy. Front Biosci 13:2293–2298
Lu Z, Kleeff J, Shrikhande S, Zimmermann T, Korc M, Friess H, Buchler MW (2000) Expression of the multidrug-resistance 1 (MDR1) gene and prognosis in human pancreatic cancer. Pancreas 21:240–247
Maeda I, Takano T, Matsuzuka F, Maruyama T, Higashiyama T, Liu G, Kuma K, Amino N (1999) Rapid screening of specific changes in mRNA in thyroid carcinomas by sequence specific-differential display: decreased expression of acid ceramidase mRNA in malignant and benign thyroid tumors. Int J Cancer 81:700–704
Mahdy AE, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, Bai A, Gault CR, McPherson AS, Garcia N, Beckham TH, Saad A, Bielawska A, Bielawski J, Hannun YA, Keane TE, Taha MI, Hammouda HM, Norris JS, Liu X (2009) Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol Ther 17:430–438
Merrill AH Jr, Sullards MC, Allegood JC, Kelly S, Wang E (2005) Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 36:207–224
Messner MC, Cabot MC (2011) Cytotoxic responses to N-(4-hydroxyphenyl)retinamide in human pancreatic cancer cells. Cancer Chemother Pharmacol 68:477–487
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Modrak DE, Leon E, Goldenberg DM, Gold DV (2009) Ceramide regulates gemcitabine-induced senescence and apoptosis in human pancreatic cancer cell lines. Mol Cancer Res 7:890–896
Morales A, Paris R, Villanueva A, Llacuna L, Garcia-Ruiz C, Fernandez-Checa JC (2007) Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene 26:905–916
Musumarra G, Barresi V, Condorelli DF, Scire S (2003) A bioinformatic approach to the identification of candidate genes for the development of new cancer diagnostics. Biol Chem 384:321–327
Park JH, Schuchman EH (2006) Acid ceramidase and human disease. Biochim Biophys Acta 1758:2133–2138
Ryland LK, Fox TE, Liu X, Loughran TP, Kester M (2011) Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther 11:138–149
Saddoughi SA, Song P, Ogretmen B (2008) Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem 49:413–440
Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A, Smyth MJ (2004) The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate 58:382–393
Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH Jr (2009) Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res 50:1692–1707
Siddique MM, Bikman BT, Wang L, Ying L, Reinhardt E, Shui G, Wenk MR, Summers SA (2012) Ablation of dihydroceramide desaturase confers resistance to etoposide-induced apoptosis in vitro. PLoS ONE 7:e44042
Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S (2010) Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol 688:141–155
Sullards MC, Allegood JC, Kelly S, Wang E, Haynes CA, Park H, Chen Y, Merrill AH Jr (2007) Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: “inside-out” sphingolipidomics. Methods Enzymol 432:83–115
Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, Symolon H, Liu Y, Merrill AH Jr, Gouaze-Andersson V, Yu JY, Giuliano AE, Cabot MC (2008) N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther 7:2967–2976
Yang K, Wu J, Li X (2008) Recent advances in the research of P-glycoprotein inhibitors. Biosci Trends 2:137–146
Zeidan YH, Jenkins RW, Korman JB, Liu X, Obeid LM, Norris JS, Hannun YA (2008) Molecular targeting of acid ceramidase: implications to cancer therapy. Curr Drug Targets 9:653–661
Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH Jr (2006) Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758:1864–1884
Acknowledgments
This work was supported by the National Cancer Institute (CA143755), the National Institute of General Medical Sciences (GM77391), and NIH GM069338 (Lipid Maps). We thank Matthew Bush for compiling the typescript and for creating the figures.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Samy A. F. Morad and Maria C. Messner contributed equally to this work.
Rights and permissions
About this article
Cite this article
Morad, S.A.F., Messner, M.C., Levin, J.C. et al. Potential role of acid ceramidase in conversion of cytostatic to cytotoxic end-point in pancreatic cancer cells. Cancer Chemother Pharmacol 71, 635–645 (2013). https://doi.org/10.1007/s00280-012-2050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-2050-4