Abstract
Purpose
The primary objective of this study was to assess the efficacy and safety of S-1 in patients with gemcitabine-resistant advanced pancreatic cancer.
Methods
Patients with histologically or cytologically proven, advanced pancreatic cancer who had received first-line chemotherapy with gemcitabine were eligible for this study. S-1 was administered orally at a dose of 40 mg/m2 twice daily for 28 days, followed by 14 days’ rest. Treatment was repeated every 6 weeks until disease progression.
Results
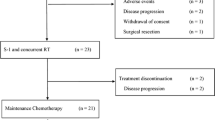
Twenty-one patients were enrolled in this study. Grade 3 and 4 toxicities included anorexia in 14% of the patients, abdominal pain in 4.8% and infection without neutropenia in 4.8%. S-1 was discontinued in two patients because of toxicity. Of the 21 eligible patients, 2 (9.5%) achieved a partial response and 9 (43%) had stable disease. A marked decrease (≥50%) in tumor marker (CA19-9) was observed in 5 (28%) of the 18 evaluable patients. The median progression-free survival and the median survival time from the first day of S-1 therapy were 4.1 months (95% CI, 1.3–6.9 months) and 6.3 months (95% CI, 3.6–8.9 months), respectively.
Conclusions
Second-line chemotherapy with S-1 was tolerated with acceptable toxicity and resulted in a relatively high disease control rate in patients with gemcitabine-resistant advanced pancreatic cancer. As an oral agent, S-1 may be a feasible treatment option for this patient population.

Similar content being viewed by others
References
Burris HA III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Pelzer U, Kubica K, Stieler J, Schwaner I, Heil G, Görner M, Mölle M, Hilbig A, Dörken B, Riess H, Oettle H (2008) A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 26(Suppl):abstr 4508
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557
Tatsumi K, Fukushima M, Shirasaka T, Fujii S (1987) Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rat liver extracts. Jpn J Cancer Res 78:748–755
Shirasaka T, Shimamoto Y, Fukushima M (1993) Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 53:4004–4009
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Shirao K, Ohtsu A, Takada H, Mitachi Y, Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H, Satoh A (2004) Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer 100:2355–2361
Kawahara M, Furuse K, Segawa Y, Yoshimori K, Matsui K, Kudoh S, Hasegawa K, Niitani H, S-1 Cooperative Study Group (Lung Cancer Working Group) (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85:939–943
Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T, Funakoshi A (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62:849–855
Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, Saito H (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Nakamura K, Yamaguchi T, Ishihara T, Sudo K, Kato H, Saisho H (2006) Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 94:1575–1579
Ueno H, Furuse J, Yamao K, Funakoshi A, Boku N, Ohkawa S, Makimoto A, Sato T, Okusaka T (2007) A multicenter phase II study of gemcitabine and S-1 combination therapy (GS therapy) in patients with metastatic pancreatic cancer. J Clin Oncol 25(Suppl):18s Abstract 4550
Tsukuda M, Kida A, Fujii M, Kono N, Yoshihara T, Hasegawa Y, Sugita M (2005) Chemotherapy study group of head and neck Cancer Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889
Morizane C, Okusaka T, Furuse J, Ishii H, Ueno H, Ikeda M, Nakachi K, Najima M, Ogura T, Suzuki E (2008) A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol 63:313–319
Burris HA III, Rivkin S, Reynolds R, Harris J, Wax A, Gerstein H, Mettinger KL, Staddon A (2005) Phase II trial of oral rubitecan in previously treated pancreatic cancer patients. Oncologist 10:183–190
Yi SY, Park YS, Kim HS, Jun HJ, Kim KH, Chang MH, Park MJ, Uhm JE, Lee J, Park SH, Park JO, Lee JK, Lee KT, Lim HY, Kang WK (2009) Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother Pharmacol 63:1141–1145
Milella M, Gelibter A, Di Cosimo S, Bria E, Ruggeri EM, Carlini P, Malaguti P, Pellicciotta M, Terzoli E, Cognetti F (2004) Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma. Cancer 101:133–138
Kim YJ, Bang S, Park JY, Park SW, Chung JB, Song SY (2009) Phase II study of 5-fluorouracil and paclitaxel in patients with gemcitabine-refractory pancreatic cancer. Cancer Chemother Pharmacol 63:529–533
Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, Hendlisz A, Van Laethem JL (2006) Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 94:481–485
Tsavaris N, Kosmas C, Skopelitis H, Gouveris P, Kopterides P, Loukeris D, Sigala F, Zorbala-Sypsa A, Felekouras E, Papalambros E (2005) Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs 23:369–375
Ulrich-Pur H, Raderer M, Verena Kornek G, Schüll B, Schmid K, Haider K, Kwasny W, Depisch D, Schneeweiss B, Lang F, Scheithauer W (2003) Irinotecan plus raltitrexed vs. raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer 88:1180–1184
Conflicts of interest statement
No financial support for this study was provided. The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed according to the guidelines of the Declaration of Helsinki as amended in Edinburgh, Scotland, in October 2000. The protocol was approved by the Institutional Review Board of Chiba University Graduate School of Medicine. All study participants provided written informed consent. This manuscript has not been published and is not under consideration for publication elsewhere.
Rights and permissions
About this article
Cite this article
Sudo, K., Yamaguchi, T., Nakamura, K. et al. Phase II study of S-1 in patients with gemcitabine-resistant advanced pancreatic cancer. Cancer Chemother Pharmacol 67, 249–254 (2011). https://doi.org/10.1007/s00280-010-1311-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1311-3