Summary
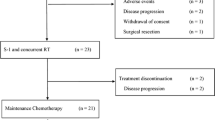
Purpose Our previous phase I trial suggested feasibility of addition of leucovorin (LV) to S-1 and gemcitabine therapy in advanced pancreatic cancer. The aim of this phase II trial was to assess the efficacy and toxicity of gemcitabine, S-1 and LV (GSL) combination therapy for advanced pancreatic cancer. Methods Chemotherapy-naïve patients with histologically or cytologically proven advanced pancreatic cancer were enrolled. Gemcitabine was administered at a dose of 1000 mg/m2 by 30 min infusion on days 1, S-1 40 mg/m2 orally twice daily and LV 25 mg orally twice daily on days 1 to 7 every 2 weeks. Primary end point was progression free survival (PFS). Results A total of 49 patients with advanced pancreatic cancer (19 locally advanced and 30 metastatic) were enrolled. Overall response rate and disease control rate were 32.7% and 87.8%. The median PFS and overall survival (OS) were 10.8 (95% confidence interval [CI], 7.4–13.5) and 20.7 (95% CI 13.0-NA) months with 1-year survival rate of 73.4%. Major Grade 3–4 toxicities were neutropenia (22.4%) and stomatitis (14.3%). No toxicity related death was observed. Conclusions In this single center, phase II trial, gemcitabine, S-1 and LV combination therapy was tolerable and can potentially be a treatment option for advanced pancreatic cancer.




Similar content being viewed by others
References
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825. https://doi.org/10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703. https://doi.org/10.1056/NEJMoa1304369
Yoshida K, Iwashita T, Uemura S, Maruta A, Okuno M, Ando N et al (2017) A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget 8(67):111346–111355. https://doi.org/10.18632/oncotarget.22795
Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J et al (2016) Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 114(7):737–743. https://doi.org/10.1038/bjc.2016.45
Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K et al (2018) A phase II study of modified FOLFIRINOX for chemotherapy-naive patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 81(6):1017–1023. https://doi.org/10.1007/s00280-018-3577-9
Nakai Y, Isayama H, Saito K, Sasaki T, Takahara N, Hamada T et al (2014) A phase I trial of gemcitabine, S-1 and LV combination (GSL) therapy in advanced pancreatic cancer. Cancer Chemother Pharmacol 74(5):911–915. https://doi.org/10.1007/s00280-014-2563-0
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England : 1990) 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
National Cancer Institute USDOHAHS (2009) Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 1 Feb 2013
Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N et al (2012) A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 106(12):1934–1939. https://doi.org/10.1038/bjc.2012.183
Ozaka M, Matsumura Y, Ishii H, Omuro Y, Itoi T, Mouri H et al (2012) Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan clinical Cancer research organization PC-01 study). Cancer Chemother Pharmacol 69(5):1197–1204. https://doi.org/10.1007/s00280-012-1822-1
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol Off J Am Soc Clin Oncol 31(13):1640–1648. https://doi.org/10.1200/JCO.2012.43.3680
Hamada C, Okusaka T, Ikari T, Isayama H, Furuse J, Ishii H et al (2017) Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. Br J Cancer 116(6):1544–1550. https://doi.org/10.1038/bjc.2017.128
Koizumi W, Boku N, Yamaguchi K, Miyata Y, Sawaki A, Kato T et al (2010) Phase II study of S-1 plus leucovorin in patients with metastatic colorectal cancer. Ann Oncol 21(4):766–771. https://doi.org/10.1093/annonc/mdp371
Ueno M, Okusaka T, Omuro Y, Isayama H, Fukutomi A, Ikeda M et al (2015) A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer. Ann Oncol 27(3):502–508. https://doi.org/10.1093/annonc/mdv603
Saito H, Watanabe Y, Sato K, Ikawa H, Yoshida Y, Katakura A et al (2014) Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer 22(11):2935–2940. https://doi.org/10.1007/s00520-014-2282-4
Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S et al (2013) Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 20(6):590–600. https://doi.org/10.1007/s00534-013-0616-0
Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB et al (2008) Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 206(5):833–846; discussion 46-8. https://doi.org/10.1016/j.jamcollsurg.2007.12.020
Rose JB, Rocha FG, Alseidi A, Biehl T, Moonka R, Ryan JA et al (2014) Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol 21(5):1530–1537. https://doi.org/10.1245/s10434-014-3486-z
Saito K, Isayama H, Sakamoto Y, Nakai Y, Ishigaki K, Tanaka M et al (2018) A phase II trial of gemcitabine, S-1 and LV combination (GSL) neoadjuvant chemotherapy for patients with borderline resectable and locally advanced pancreatic cancer. Med Oncol 35(7):100. https://doi.org/10.1007/s12032-018-1158-8
Chung KH, Ryu JK, Lee BS, Jang DK, Lee SH, Kim YT (2016) Early decrement of serum carbohydrate antigen 19-9 predicts favorable outcome in advanced pancreatic cancer. J Gastroenterol Hepatol 31(2):506–512. https://doi.org/10.1111/jgh.13075
Nakai Y, Kawabe T, Isayama H, Sasaki T, Yagioka H, Yashima Y et al (2008) CA 19-9 response as an early indicator of the effectiveness of gemcitabine in patients with advanced pancreatic cancer. Oncology 75(1–2):120–126. https://doi.org/10.1159/000155213
Chiorean EG, Von Hoff DD, Reni M, Arena FP, Infante JR, Bathini VG et al (2016) CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine vs gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 27(4):654–660. https://doi.org/10.1093/annonc/mdw006
Acknowledgments
The authors would like to thank Drs. Dai Akiyama, Kaoru Takagi, Takeo Watanabe, Ryouta Takahashi, Dai Mohri and Kenji Hirano for their patient management.
Funding
This research was supported by Japanese foundation for multidisciplinary treatment of cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyuki Isayama and Yousuke Nakai received research funding from Taiho Pharmaceutical Co. All remaining authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethical Committee of the University of Tokyo Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Saito, K., Isayama, H., Nakai, Y. et al. A phase II trial of gemcitabine, S-1 and LV combination (GSL) therapy in patients with advanced pancreatic cancer. Invest New Drugs 37, 338–344 (2019). https://doi.org/10.1007/s10637-018-0691-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0691-9