Abstract
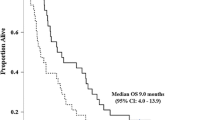
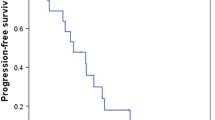
A pilot phase II study showed S-1 monotherapy to be safe and active against biliary tract cancer (BTC). We, therefore, conducted a multicenter phase II study to evaluate the antitumor effect and safety of S-1 in previously untreated patients with advanced BTC. Eligible patients had pathologically proven, unresectable adenocarcinoma with no prior chemotherapy or radiotherapy. Patients received S-1 orally at 80 mg/m2 total daily dose divided b.i.d. for 28 days followed by 14 days of rest. Of the 41 enrolled patients, 40 were assessable. The primary tumor sites were as follows: gallbladder (n = 20), extrahepatic bile duct (n = 15), and the ampulla of Vater (n = 5). One patient (2.5%) achieved a complete response, 13 patients (32.5%) had partial responses, 17 patients (42.5%) had no change, 7 patients (17.5%) had progressive disease, and 2 patients (5.0%) were not evaluable. The overall objective response rate was 35.0%. The median overall survival (median OS) was 9.4 months, and the median time to progression was 3.7 months. Grade 3 or 4 toxicities included fatigue (7.5%), anorexia (7.5%) and T-Bil elevation (7.5%). Significant antitumor activity combined with a mild toxicity profile was observed. This monotherapy warrants further evaluation in a randomized study.



Similar content being viewed by others
References
Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D et al (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 15(9):1339–1343
Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S et al (2004) A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer 90(8):1516–1520
Falkson G, MacIntyre JM, Moertel CG (1984) Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 54(6):965–969
Furuse J, Okusaka T, Funakoshi A, Boku N, Yamao K, Ohkawa S et al (2005) A phase II study of S-1 in patients with metastatic pancreatic cancer. Proc Am Soc Clin Oncol 23:16s (Abstract No: 4104)
Furuse J, Okusaka T, Funakoshi A, Yamao K, Nagase M, Ishii H et al (2006) Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol 36(9):552–556
Gallardo JO, Rubio B, Fodor M, Orlandi L, Yanez M, Gamargo C et al (2001) A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol 12(10):1403–1406
Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK et al (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7(6):593–600
Haskell CM (ed) (2001) Cancer treatment, 5th edn. WB Saunders Co, Philadelphia
Hejna M, Pruckmayer M, Raderer M (1998) The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer 34(7):977–986
Ikeda M, Okusaka T, Ueno H, Morizane C, Furuse J, Ishii H (2005) A phase II trial of Uracil-tegafur (UFT) in patients with advanced biliary tract carcinoma. Jpn J Clin Oncol 35(8):439–443
Kawahara M, Furuse K, Segawa Y, Yoshimori K, Matsui K, Kudoh S et al (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85(7):939–943
Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH et al (2003) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 14(7):1115–1120
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E et al (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23(10):2332–2338
Knox JJ, Hedley D, Oza A, Siu LL, Pond GR, Moore MJ (2004) Gemcitabine concurrent with continuous infusional 5-fluorouracil in advanced biliary cancers: a review of the Princess Margaret Hospital experience. Ann Oncol 15(5):770–774
Koyama Y (ed) (1993) Criteria for the evaluation of the clinical effects of solid cancer chemotherapy. J Jpn Soc Cancer Ther 28:101–130
Levin B (1999) Gallbladder carcinoma. Ann Oncol 10(Suppl 4):129–130
Lin MH, Chen JS, Chen HH, Su WC (2003) A phase II trial of gemcitabine in the treatment of advanced bile duct and periampullary carcinomas. Chemotherapy 49(3):154–158
Makuuchi M (ed) (2000) The general rules for the clinical and pathological study of primary liver cancer November 2000, 4th edn. Kanehara, Tokyo
Mani S, Sciortino D, Samuels B, Arrietta R, Schilsky RL, Vokes EE et al (1999) Phase II trial of uracil/tegafur (UFT) plus leucovorin in patients with advanced biliary carcinoma. Invest New Drugs 17(1):97–101
Nagakawa T (ed) (2003) General rules for surgical and pathological studies on cancer of the biliary tract September 2003, 5th edn. Kanehara, Tokyo
Nomura K (ed) (2005) Cancer statistics in Japan 2005. Foundation for Promotion of Cancer Research (FPCR), Tokyo
Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K et al (2000) Phase II study of S-1, a novel oral fluorophyrimidine derivative, in patients with metastatic colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study Group. Br J Cancer 83(2):141–145
Okusaka T, Ishii H, Funakoshi A, Yamao K, Ohkawa S, Saito S et al (2006) Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 57(5):647–653
Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA et al (2004) Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 101(3):578–586
Patt YZ, Jones DV Jr, Hoque A, Lozano R, Markowitz A, Raijman I et al (1996) Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol 14(8):2311–2315
Saeki T, Takashima S, Sano M, Horikoshi N, Miura S, Shimizu S et al (2004) A phase II study of S-1 in patients with metastatic breast cancer—a Japanese trial by the S-1 Cooperative Study Group, Breast Cancer Working Group. Breast Cancer 11(2):194–202
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34(11):1715–1720
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7(5):548–557
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Ueno H, Furuse J, Yamao K, Funakoshi A, Boku N, Ohkawa S et al (2007) A multicenter phase II study of gemcitabine and S-1 combination therapy (GS therapy) in patients with metastatic pancreatic cancer. ASCO 2007 Gastrointestinal Cancers Symposium (Abstract No: 148)
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C (2004) Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 91(10):1769–1774
van Kuilenburg AB, De Abreu RA, van Gennip AH (2003) Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem 40(Pt 1):41–45
Acknowledgments
We thank Drs. M. Kurihara, S. Matsuno, O. Ishikawa, C. Hamada and T. Taguchi for their kind advice, and Drs. H. Saisho, N. Moriyama and W. Koizumi for this extramural review. The authors are indebted to Professor J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University for his review of this manuscript. This study was supported by Taiho Pharmaceutical Co. Ltd.
Conflict of interest
The study was supported by Taiho Pharmaceutical Co., Ltd. (Tokyo). For all authors, there is no potential conflict of interest, relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furuse, J., Okusaka, T., Boku, N. et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62, 849–855 (2008). https://doi.org/10.1007/s00280-007-0673-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0673-7