Abstract
Background
We aimed to determine the maximum-tolerated dose (MTD) of S-1 when given with oxaliplatin, to evaluate S-1 pharmacokinetics, and to determine the efficacy and safety of this regimen as a first-line treatment for advanced gastric cancer (AGC).
Methods
Oxaliplatin was fixed at a dose of 130 mg/m2 on day 1 (D1). S-1 was administered from D1 to D14 of a 3-week cycle, and escalated by 10 mg/m2 per day from 70 mg/m2 per day up to 100 mg/m2 per day. Pharmacokinetic analyses were performed following a single dose of S-1 on D-5 and D1 of the first cycle.
Results
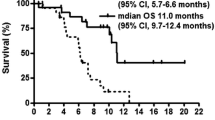
In phase I (n = 18), MTD was not defined. In phase II (n = 47) with the planned maximum dose, partial response was achieved in 26 patients (55.3%) and stable disease in 14 patients (29.8%). The median time to progression was 6.6 months (95% CI 4.0–9.2 months) and the median overall survival was 12.5 months (95% CI 9.2–15.9 months). Frequent grade 3/4 toxicities included thrombocytopenia (39%), neutropenia (28%), anemia (17%), and leukopenia (13%). There was one grade 5 febrile neutropenia during the first cycle.
Conclusions
The pharmacokinetics of S-1 was not influenced by oxaliplatin. S-1/Oxaliplatin combination therapy is highly active against AGC and has a favorable toxicity profile.


Similar content being viewed by others
References
Parkin D, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Rivera F, Vega-Villegas M, Lopez-Brea M (2007) Chemotherapy of advanced gastric cancer. Cancer Treat Rev 33:315–324
Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden P, Haglund U, Svensson C, Enander L, Linne T, Sellstrom H, Heuman R (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8:163–168
Murad A, Santiago F, Petroianu A, Rocha P, Rodrigues M, Rausch M (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72:37–41
Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71:587–591
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman A (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Kang Y, Kang W, Shin D, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud P (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Chollet P, Schoffski P, Weigang-Kohler K, Schellens J, Cure H, Pavlidis N, Grunwald V, De BR, Wanders J, Fumoleau P (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39:1264–1270
Koizumi W, Kurihara M, Nakano S, Hasegawa K (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58:191–197
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Sugimachi K, Maehara Y, Horikoshi N, Shimada Y, Sakata Y, Mitachi Y, Taguchi T (1999) An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. The S-1 Gastrointestinal Cancer Study Group. Oncology 57:202–210
Araki H, Fukushima M, Kamiyama Y, Shirasaka T (2000) Effect of consecutive lower-dose cisplatin in enhancement of 5-fluorouracil cytotoxicity in experimental tumor cells in vivo. Cancer Lett 160:185–191
Ajani J, Faust J, Ikeda K, Yao J, Anbe H, Carr K, Houghton M, Urrea P (2005) Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 23:6957–6965
Ajani J, Lee F, Singh D, Haller D, Lenz H, Benson Ar, Yanagihara R, Phan A, Yao J, Strumberg D (2006) Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24:663–667
Hyodo I, Nishina T, Moriwaki T, Endo S, Terao T, Hirao K, Nasu J, Hirasaki S, Endo H, Masumoto T, Tajiri H, Kurita A (2003) A phase I study of S-1 combined with weekly cisplatin for metastatic gastric cancer in an outpatient setting. Eur J Cancer 39:2328–2333
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M (2003) Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 89:2207–2212
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Di FA, Ruggiero A, Riccardi R (2002) Cellular and molecular aspects of drugs of the future: oxaliplatin. Cell Mol Life Sci 59:1914–1927
Eriguchi M, Nonaka Y, Yanagie H, Yoshizaki I, Takeda Y, Sekiguchi M (2003) A molecular biological study of anti-tumor mechanisms of an anti-cancer agent oxaliplatin against established human gastric cancer cell lines. Biomed Pharmacother 57:412–415
Al-Batran S, Hartmann J, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs H, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jager E (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 26:1435–1442
Caussanel J, Levi F, Brienza S, Misset J, Itzhaki M, Adam R, Milano G, Hecquet B, Mathe G (1990) Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst 82:1046–1050
Therasse P, Arbuck S, Eisenhauer E, Wanders J, Kaplan R, Rubinstein L, Verweij J, Van GM, van OA, Christian M, Gwyther S (2000) New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Maehara Y (2003) S-1 in gastric cancer: a comprehensive review. Gastric Cancer 6(Suppl 1):2–8
MacDonald J, Schein P, Woolley P, Smythe T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet R, Lagarde C (1980) 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med 93:533–536
Park Y, Lee J, Ryoo B, Ryu M, Yang S, Kim B, Shin D, Chang H, Kim T, Yuh Y, Kang Y (2008) Capecitabine in combination with oxaliplatin (XELOX) as a first-line therapy for advanced gastric cancer. Cancer Chemother Pharmacol 61:623–629
Lee J, Kang H, Kang Y, Ryu M, Chang H, Kim T, Sohn H, Kim H, Lee J (2008) Phase I/II study of 3-week combination of S-1 and cisplatin chemotherapy for metastatic or recurrent gastric cancer. Cancer Chemother Pharmacol 61:837–845
Kim T, Kang Y, Ahn J, Chang H, Yook J, Oh S, Kim B, Lee J (2002) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 13:1893–1898
Lee J, Kang Y, Kang H, Lee K, Zang D, Ryoo B, Kim J, Park S, Kang W, Shin D, Ryu M, Chang H, Kim T, Baek J, Min Y (2008) A randomised multicentre phase II trial of capecitabine versus S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 99:584–590
Nagashima F, Ohtsu A, Yoshida S, Ito K (2005) Japanese nationwide post-marketing survey of S-1 in patients with advanced gastric cancer. Gastric Cancer 8:6–11
Kimura Y, Kikkawa N, Iijima S, Kato T, Naoi Y, Hayashi T, Tanigawa T, Yamamoto H, Kurokawa E (2003) A new regimen for S-1 therapy aiming at adverse reaction mitigation and prolonged medication by introducing a 1-week drug-free interval after each 2-week dosing session: efficacy and feasibility in clinical practice. Gastric Cancer 6(Suppl 1):34–39
Zhu A, Clark J, Ryan D, Meyerhardt J, Enzinger P, Earle C, Fuchs C, Regan E, Anbe H, Houghton M, Zhang J, Urrea P, Kulke M (2007) Phase I and pharmacokinetic study of S-1 administered for 14 days in a 21-day cycle in patients with advanced upper gastrointestinal cancer. Cancer Chemother Pharmacol 59:285–293
Tsukuda M, Kida A, Fujii M, Kono N, Yoshihara T, Hasegawa Y, Sugita M (2005) Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889
Yamada Y, Tahara M, Miya T, Satoh T, Shirao K, Shimada Y, Ohtsu A, Sasaki Y, Tanigawara Y (2008) Phase I/II study of oxaliplatin with oral S-1 as first-line therapy for patients with metastatic colorectal cancer. Br J Cancer 98:1034–1038
Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1999) Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5:2000–2005
Kim W, Nakata B, Hirakawa K (2007) Alternative pharmacokinetics of S-1 components, 5-fluorouracil, dihydrofluorouracil and alpha-fluoro-beta-alanine after oral administration of S-1 following total gastrectomy. Cancer Sci 98:1604–1608
Acknowledgments
We thank to Jeil Pharmaceutical Company for donation of S-1 and Sanofi Aventis for donation of oxaliplatin. We are indebted to Taiho Pharmaceutical Company for pharmacokinetic study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, I., Lee, JL., Ryu, MH. et al. Phase I/II and pharmacokinetic study of S-1 and oxaliplatin in previously untreated advanced gastric cancer. Cancer Chemother Pharmacol 65, 473–480 (2010). https://doi.org/10.1007/s00280-009-1052-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1052-3