Abstract
Purpose
To define the maximum-tolerated dose (MTD) of S-1, given daily for 2 weeks followed by a 1-week rest, with a fixed dose of cisplatin on the initial day, and to determine the activity and safety of this regimen at the recommended dose (RD) when used as first line treatment of advanced gastric cancer (AGC).
Patients and methods
Cisplatin was fixed at a dose of 60 mg/m2 on day 1 (D1) and the starting dose of S-1 was 60 mg/m2/day (30 mg/m2 bid) (level I) on D1 to D14, every 3 weeks. The dose of S-1 was increased by 5 mg/m2 bid up to 100 mg/m2/day (level V) unless the MTD was achieved.
Results
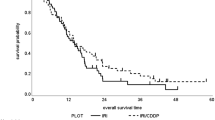
Sixty-two eligible patients were enrolled. MTD was set at level V with two of three patients developing grade 3 diarrhea or febrile neutropenia. The RD was determined at level IV (90 mg/m2/day). After the first 20 patients were enrolled in phase II, the protocol was amended; the S-1 dose was reduced to 80 mg/m2/day (N = 23) because of poor bone marrow recovery. The objective response was observed in 20 of 42 evaluable patients (48%). SD was achieved in 15 (36%). The median PFS was 5.3 months (95% CI, 4.6–6.0 months) with a median OS of 10.0 months (95% CI, 5.1–14.8 months). Grade 3–4 toxicities included neutropenia (33%), anemia (31%), and anorexia (24%).
Conclusions
The 3-week combination of cisplatin plus S-1 is active against AGC with favorable toxicitiy profiles. The phase II schedule or doses may need further refinements.


Similar content being viewed by others
References
Ajani JA, Faust J, Ikeda K, Yao JC, Anbe H, Carr KL, Houghton M, Urrea P (2005) Phase I Pharmacokinetic Study of S-1 Plus Cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 23:6957–6965
Ajani JA, Lee F-C, Singh DA, Haller DG, Lenz H-J, Benson AB III, Yanagihara R, Phan AT, Yao JC, Strumberg D (2006) Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24:663–667
Araki H, Fukushima M, Kamiyama Y, Shirasaka T (2000) Effect of consecutive lower-dose cisplatin in enhancement of 5-fluorouracil cytotoxicity in experimental tumor cells in vivo. Cancer Lett 160:185–91
Baba H, Yamamoto M, Endo K, Ikeda Y, Toh Y, Kohnoe S, Okamura T (2003) Clinical efficacy of S-1 combined with cisplatin for advanced gastric cancer. Gastric Cancer 1(6 Suppl ):45–9
Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ (2004) Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 22:2395–2403
Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau P (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC early clinical studies group (ECSG). Eur J Cancer 39:1264–1270
Cohen SJ, Leichman CG, Yeslow G, Beard M, Proefrock A, Roedig B, Damle B, Letrent SP, DeCillis AP, Meropol NJ (2002) Phase I and pharmacokinetic study of once daily oral administration of S-1 in patients with advanced cancer. Clin Cancer Res 8:2116–2122
Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1999) Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5:2000–2005
Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, Dwivedy S, Russo M, Pazdur R (2003) Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res 9:134–142
Hyodo I, Nishina T, Moriwaki T, Endo S, Terao T, Hirao K, Nasu J, Hirasaki S, Endo H, Masumoto T, Tajiri H, Kurita A (2003) A phase I study of S-1 combined with weekly cisplatin for metastatic gastric cancer in an outpatient setting. Eur J Cancer 39:2328–2333
Kang Y, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Philco M, Suarez T, Santamaria J (2006) Randomized phase III trial of capecitabine/cisplatin (XP) vs. continuous infusion of 5-FU/cisplatin (FP) as first-line therapy in patients (pts) with advanced gastric cancer (AGC): Efficacy and safety results. J Clin Oncol (Meeting Abstracts) 24:LBA4018-
Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ et al. (1993) A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 71:3813–3818
Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, Kim BS, Lee JS (2002) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 13:1893–1898
Kimura Y, Kikkawa N, Iijima S, Kato T, Naoi Y, Hayashi T, Tanigawa T, Yamamoto H, Kurokawa E (2003) A new regimen for S-1 therapy aiming at adverse reaction mitigation and prolonged medication by introducing a 1-week drug-free interval after each 2-week dosing session: efficacy and feasibility in clinical practice. Gastric Cancer 6(Suppl 1):34–39
Koizumi W, Kurihara M, Nakano S, Hasegawa K (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 cooperative gastric cancer study group. Oncology 58:191–197
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M (2003) Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 89:2207–2212
Kusuoka H, Hoffman JI (2002) Advice on statistical analysis for circulation research. Circ Res 91:662–71
Nagashima F, Ohtsu A, Yoshida S, Ito K (2005) Japanese nationwide post-marketing survey of S-1 in patients with advanced gastric cancer. Gastric Cancer 8:6–11
Nakata B, Mitachi Y, Tsuji A, Yamamitsu S, Hirata K, Shirasaka T, Hirakawa K (2004) Combination phase I trial of a novel oral fluorouracil derivative S-1 with low-dose cisplatin for unresectable and recurrent gastric cancer (JFMC27-9902). Clin Cancer Res 10:1664–1669
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, Price T, Anderson H, Iveson T, Hickish T, Lofts F, Norman A (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Sato Y, Kondo H, Honda K, Takahari D, Sumiyoshi T, Tsuji Y, Yoshizaki N, Niitsu Y (2005) A phase I/II study of S-1 plus cisplatin in patients with advanced gastric cancer: 2-week S-1 administration regimen. Int J Clin Oncol 10:40–44
Shimada T, Yamazaki H, Guengerich FP (1996) Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica 26:395–403
Shimoyama S, Imamura K, Hiki N, Yamaguchi H, Mafune K, Kaminishi M (2005) Performance of outpatient regimen of S-1 in combination with fractional cisplatin for advanced or recurrent gastric cancers: a phase I study. Int J Clin Oncol 10:251–255
Tsukuda M, Kida A, Fujii M, Kono N, Yoshihara T, Hasegawa Y, Sugita M (2005) Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse M-L, Ajani JA (2006) Phase III Study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J C65lin Oncol 24:4991–4997
Van den Brande J, Schoffski P, Schellens JH, Roth AD, Duffaud F, Weigang-Kohler K, Reinke F, Wanders J, de Boer RF, Vermorken JB, Fumoleau P (2003) EORTC early clinical studies group early phase II trial of S-1 in patients with advanced or metastatic colorectal cancer. Br J Cancer 88:648–653
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JGD, Piedbois P, Paillot B, Bodenstein H, Schmoll H-J, Bleiberg H, Nordlinger B, Couvreur M-L, Baron B, Wils JA (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the european organization for research and treatment of cancer gastrointestinal tract cancer cooperative group. J Clin Oncol 18:2648–2657
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O’Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15:261–267
Zhu AX, Clark JW, Ryan DP, Meyerhardt JA, Enzinger PC, Earle CC, Fuchs CS, Regan E, Anbe H, Houghton M, Zhang J, Urrea P, Kulke MH (2007) Phase I and pharmacokinetic study of S-1 administered for 14 days in a 21-day cycle in patients with advanced upper gastrointestinal cancer. Cancer Chemother Pharmacol 59:285–293
Acknowledgment
This study was supported in part by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A060775). S-1 was provided by Jeil Pharmacetucial Co. Ltd. Seoul, Korea. This study was presented in part at the 2007 ASCO Gastrointestinal Cancer Symposium, Orlando, Fl, USA., January 19–21, 2007. The authors are indebted to Professor J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University and Professor Jaffer A. Ajani of the University of Texas M. D. Anderson Cancer Center for their review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JL., Kang, H.J., Kang, YK. et al. Phase I/II study of 3-week combination of S-1 and cisplatin chemotherapy for metastatic or recurrent gastric cancer. Cancer Chemother Pharmacol 61, 837–845 (2008). https://doi.org/10.1007/s00280-007-0541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0541-5