Abstract
Purpose
S-1 is a novel oral fluoropyrimidine that combines tegafur with CDHP and oxonic acid. To decrease the incidence of late onset, severe diarrhea observed in a previous study, a phase I study was conducted to determine the maximum tolerated dose (MTD) of S-1 utilizing a 14-day schedule, repeated every 21 days, in patients with chemotherapy–refractory upper gastrointestinal malignancies.
Methods
S-1 was administered orally, twice-daily, at an initial dose level of 30 mg/m2/dose; doses were escalated by 5 mg/m2 at each level. A minimum of three patients were enrolled at each dose level. S-1 toxicity, antitumor activity, and pharmacokinetics were assessed. The MTD was based on the dose limiting toxicity (DLT) during the first treatment cycle.
Results
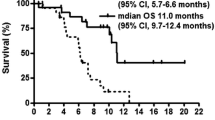
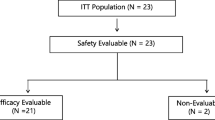
At 30 mg/m2 no DLT was observed in the first three evaluable patients. Two of the first three patients at the 35 mg/m2 dose level developed DLTs (grade 3 rash and dehydration). An additional nine patients were subsequently treated at 30 mg/m2 without DLT and this dose was established as the MTD. Common toxicities at 30 mg/m2 included diarrhea, nausea, skin rash, anorexia, and fatigue. No grade 4 toxicities were observed. One partial response was seen in a patient with gemcitabine-refractory pancreatic adenocarcinoma and ten patients with pancreatic, gastric, or gallbladder carcinomas achieved stable disease as their best response to therapy. The AUC(0–8) of 5-FU at the 30 and 35 mg/m2 dose levels were 875 ± 212 and 894 ± 151 h ng/ml, respectively.
Conclusions
In a 14-day dosing schedule, the MTD of S-1 was 30 mg/m2 and preliminary evidence of antitumor activity was seen in a North American population with refractory upper gastrointestinal malignancies.

Similar content being viewed by others
References
Ajani JA, Faust J, Ikeda K, Yao JC, Anbe H, Carr KL, Houghton M, Urrea P (2005) Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 23:6957–6965
Ajani JA, Lee F-C, Singh DA, Haller DG, Lenz H-J, Benson III AB, Yanagihara R, Phan AT, Yao JC, Strumberg D (2006) Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24:663–667
Cancer Therapy Evaluation Program (2003) Common terminology criteria for adverse advents. DCTD. NCI. NIH. DHHS, V.3.0. http://www.ctep.cancer.gov. Cited 10 June 2003
Chollet P, Schöffski P, Weigang-Köhler K, Schellens JHM, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39:1264–1270
Cocconi G, Carlini P, Gamboni A, Gasperoni S, Rodino C, Zironi S, Bisagni G, Porrozzi S, Cognetti F, Di Costanzo F, Canaletti R, Ruggeri EM, Camisa R, Pucci F (2003) Cisplatin, epirubicin, leucovorin and 5-fluorouracil (PELF) is more active than 5-fluorouracil, doxorubicin and methotrexate (FAMTX) in advanced gastric carcinoma. Ann Oncol 14:1258–1263
Cohen SJ, Leichman CG, Yeslow G, Beard M, Proefrock A, Roedig B, Damle B, Letrent SP, DeCillis AP, Meropol NJ (2002) Phase I and pharmacokinetic study of once daily oral administration of S-1 in patients with advanced cancer. Clin Cancer Res 8:2116–2122
Fujii M, Endo S, Tomita K, Nishijima W, Tsukuda M, Hasegawa Y, Ishitoya J, Yamane H, Fujii H, Honma A, Tomita T (2005) A phase I/II study of S-1 plus cisplatin (CDDP) in patients with head and neck cancer (HNC) (Meeting Abstracts). J Clin Oncol 23:5552
Furuse K, Kawahara M, Hasegawa K, Kudoh S, Takada M, Sugiura T, Ichinose Y, Fukuoka M, Ohashi Y, Niitani H, for the S-1 Cooperative Study Group (Lung Cancer Working Group) (2001) Early phase II study of S-1, a new oral fluoropyrimidine, for advanced non-small-cell lung cancer. Int J Clin Oncol 6:236–241
Furuse J, Okusaka T, Funakoski A, Boku N, Yamao K, Ohkawa S, Saito H (2005) A phase II study of S-1 in patients with metastatic pancreatic cancer (Meeting Abstracts). J Clin Oncol 23(16S):4104
Hino M, Saeki T, Sato Y, Sano M (2004) Late phase II study of S-1 in patients with taxane resistant breast cancer (Meeting Abstracts). J Clin Oncol 22:745
Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1999) Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 5:2000–2005
Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, Dwivedy S, Russo M, Pazdur R (2003) Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res 9:134–142
Ichinose Y, Yoshimori K, Sakai H, Nakai Y, Sugiura T, Kawahara M, Niitani H (2004) S-1 plus cisplatin combination chemotherapy in patients with advanced non-small cell lung cancer: a multi-institutional phase II trial. Clin Cancer Res 10:7860–7864
Ikeda K, Yoshisue K, Matsushima E, Nagayama S, Kobayashi K, Tyson CA, Chiba K, Kawaguchi Y (2000) Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res 6:4409–4415
Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B (1998) Early phase II study of S-1 in patients with advanced head and neck cancer. Jpn J Cancer Chemother 25:1151–1158
Kajita J, Fuse E, Kuwabara T, Kobayashi H (2003) The contribution of cytochrome P450 to the metabolism of tegafur in human liver. Drug Metab Pharmacokinet 18:303–309
Kawahara M, Furuse K, Segawa Y, Yoshimori K, Matsul K, Kudoh S, Hasegawa K, Niitani H, S-1 Cooperative Study Group (Lung Cancer Working Group) (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Brit J Cancer 85:939–943
Koizumi W, Kurihara M, Nakano S, Hasegawa K, for the S-1 Cooperative Gastric Cancer Study Group (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. Oncology 58:191–197
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M (2003) Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Brit J Cancer 89:2207–2212
Matsushima E, Yoshida K, Kitamura R, Yoshida K (1997) Determination of S-1 (combined drug tegafur, 5-chloro-2,4-dihydroxypyridine and potassium oxonate) and 5-fluorouracil in human plasma and urine using high-performance liquid chromatography and gas chromatography negative ion chemical ionization mass spectrometry. J Chromatogr B Biomed Sci Appl 691:95–104
Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, Taguchi T, for the S-1 Cooperative Colorectal Carcinoma Study Group (2000) Phase II study of S-1, a novel oral fluorophyrimidine derivative, in patients with metastatic colorectal carcinoma. Brit J Cancer 83:141–145
Peters GJ, Noordhuis P, van Groeningen CJ, Giaccone G, Holwerda U, Voorn D, Schrijvera A, Schornagel JH, Beijnen JH, Fumoleau P, Schellens JHM (2004) The effect of food on the pharmacokinetics of S-1 after single oral administration to patients with solid tumors. Clin Cancer Res 10:4072–4076
Rao S, Cunningham D, Hawkins RE, Hill ME, Smith D, Daniel F, Ross PJ, Oates J, Norman AR (2005) Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Brit J Cancer 92:1650–1654
Rani M, Cordio S, Milandri C, Passoni P, Bonetto E, Oliani C, Luppi G, Nicoletti R, Galli L, Bordonaro R (2005) Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: a randomised controlled multicrentre phase III trial. Lancet Oncol 6:369–376
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34:1715–1720
Sano M, Saeki T, Takashima S, Horikoshi N, Miura S, Morimoto K, Noguchi S, Taguchi T (2000) Late phase II study of S-1 in patients with advanced and/or recurrent breast cancer (Meeting Abstracts). J Clin Oncol 18:404
Shimada T, Yamazaki H, Guengerich FP (1996) Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica 26:395–403
Shirao K, Ohtsu A, Takada H, Mitachi Y, Hakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H, Satoh A (2004) Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer 100:2355–2361
Shirasaka T, Shimamoto Y, Fukushima M (1993) Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 53:4004–4009
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anti-Cancer Drugs 7:548–557
Sugimachi K, Maehara Y, Horikoshi N, Shimada Y, Sakata Y, Mitachi Y, Taguchi T, The S-1 Gastrointestinal Cancer Study Group (1999) An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. Oncology 57:202–210
Taguchi T, Morimoto K, Horikoshi N, Takashima S, Toge T, Kimura M, Sano M, Aoyama H, Ota J, Noguchi S (1998) An early phase II clinical study of S-1 in patients with breast cancer. S-1 Cooperative Study Group (Breast Cancer Working Group). Jpn J Cancer Chemother 25:1035–1043
Takechi T, Nakano K, Uchida J, Mita A, Toko K, Takeda S, Unemi N, Shirasaka T (1997) Antitumor activity and low intestinal toxicity of S-1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol 39:205–211
Tatsumi K, Fukushima M, Shirasaka T, Fujii S (1987) Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rat liver extracts. Jpn J Cancer Res (Gann) 78:748–755
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C (2004) Phase II study of S-1 in patents with advanced biliary tract cancer. Brit J Cancer 91:1769–1774
Van den Brande J, Schöffski P, Schellens JHM, Roth AD, Duffaud F, Weigang-Kohler K, Reinke F, Wanders J, de Boer RF, Vermorken JB, Fumoleau P (2003) EORTC Early Clinical Studies Group early phase II trial of S-1 in patients with advanced or metastatic colorectal cancer. Brit J Cancer 88:648–653
Groeningen CJ, Peters GJ, Schornagel JH, Gall H, Noordhuis P, de Vries MJ, Turner SL, Swart MS, Pinedo HM, Hanauske AR, Giaccone G (2000) Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol 18:2772–2779
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Dos Santos JG, Piedbois P, Paillot B, Bodenstein H, Schmoll H-J, Bleiberg H, Nordlinger B, Couvreur M-L, Baron B, Wils JA (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European organization for research and treatment of cancer gastrointestinal tract cancer cooperative group. J Clin Oncol 18:2648–2657
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Jaffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O'Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15:261–267
Acknowledgments
We thank Lukas Makris, John Ilgenfritz, Susan Cousounis, Peter Nicola, and Kathrin Krakauer for data analysis and manuscript review at BioCor, Yardley, PA. We thank Taylor S. Spear for assistance in the preparation and editing of this manuscript. M.H. Kulke is supported in part by NIH grants K23 CA 093401 and K30 HL04095.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported in part by a grant from Taiho Pharma USA Inc., Princeton, NJ, USA.
Rights and permissions
About this article
Cite this article
Zhu, A.X., Clark, J.W., Ryan, D.P. et al. Phase I and pharmacokinetic study of S-1 administered for 14 days in a 21-day cycle in patients with advanced upper gastrointestinal cancer. Cancer Chemother Pharmacol 59, 285–293 (2007). https://doi.org/10.1007/s00280-006-0265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0265-y