Abstract
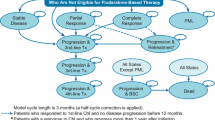
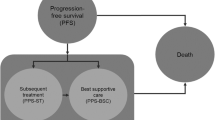
Though the chronic lymphocytic leukaemia (CLL) management options in India are still limited compared to the novel drug options in resource-rich settings, the availability of less costly generics and the government health insurance scheme has enabled many patients to access the newer drugs in India. The current study compared the cost-effectiveness and cost-utility of existing initial management options for the progression-free survival (PFS) time horizon from the patient’s perspective. A two-health-state, PFS and progressive disease, Markov model was assumed for three regimens (generics): ibrutinib monotherapy, bendamustine-rituximab (B-R), and rituximab-chlorambucil (RClb) used as the frontline treatment of CLL patients in India. All costs, utilization of services, and consequences data during the PFS period were collected from interviewing patients during follow-up visits. The transition probability (TP) and average PFS information were obtained from landmark published studies. EQ-5D-5L questionnaires were utilized to assess the quality of life (QoL). Quality-adjusted life years (QALY) were measured during the PFS period. The incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR) were studied. Upon analysis, the entire monetary expense during the PFS time was ₹1581964 with ibrutinib, ₹171434 with B-R, and ₹91997 with RClb treatment arm. Pooled PFS and QALY gain was 10.33 and 8.28 years for ibrutinib, 4.08 and 3.53 years for the B-R regimen, and 1.33 and 1.23 years in RClb arms, respectively. Ibrutinib’s ICER and ICUR were ₹214587.32 per PFS year gain and ₹282384.86 per QALY gain when assessed against the B-R regimen. Ibrutinib also performed better in ICER and ICUR against the RClb arm with ₹157014.29 per PFS year gain and ₹200413.6 per QALY gain. In conclusion, generic ibrutinib is a cost-effective initial line of management compared to other commonly used treatment regimes in resource-limited settings.





Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available to preserve study participant privacy and are available from the corresponding author upon reasonable request.
References
Yao Y, Lin X, Li F, Jin J, Wang H (2022) The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: analysis based on the global burden of disease study 2019. Biomed Eng Online 21(1):4
Tejaswi V, Lad DP, Jindal N, Prakash G, Malhotra P, Khadwal A et al (2020) Chronic lymphocytic leukemia: real-world data from India. JCO Glob Oncol 6:866–872
Todorovic Z, Todorovic D, Markovic V, Ladjevac N, Zdravkovic N, Djurdjevic P et al (2022) CAR T cell therapy for chronic lymphocytic leukemia: successes and shortcomings. Curr Oncol 29(5):3647–3657
Angell BJ, Prinja S, Gupt A, Jha V, Jan S (2019) The Ayushman Bharat Pradhan Mantri Jan Arogya Yojana and the path to universal health coverage in India: overcoming the challenges of stewardship and governance. PLoS Med 16(3):e1002759
Sriee GVVP, Maiya GR (2021) Coverage, utilization, and impact of Ayushman Bharat scheme among the rural field practice area of Saveetha Medical College and Hospital, Chennai. J Family Med Prim Care 10(3):1171–1176
Youron P, Singh C, Jindal N, Malhotra P, Khadwal A, Jain A et al (2020) Quality of life in patients of chronic lymphocytic leukemia using the EORTC QLQ-C30 and QLQ-CLL17 questionnaire. Eur J Haematol 105(6):755–762
Singh C, Jindal N, Youron P, Malhotra P, Prakash G, Khadwal A et al (2021) Efficacy, safety, and quality of life of generic and innovator ibrutinib in Indian CLL patients. Indian J Hematol Blood Transfus 37(2):313–317
Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A et al (2020) Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 34(3):787–798
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C et al (2016) First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 17(7):928–942
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al (2014) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370(12):1101–1110
India GDP per capita (2021) StatisticsTimes.com [Internet]. [Cited 2023 Apr 27]. https://statisticstimes.com/economy/country/india-gdp-per-capita.php
Blankart CR, Koch T, Linder R, Verheyen F, Schreyögg J, Stargardt T (2013) Cost of illness and economic burden of chronic lymphocytic leukemia. Orphanet J Rare Dis 20(8):32
Huang Q, Emond B, Lafeuille MH, Gupta D, Lefebvre P, Sundaram M et al (2020) Healthcare resource utilization and costs associated with first-line ibrutinib compared to chemoimmunotherapy treatment among Medicare beneficiaries with chronic lymphocytic leukemia. Curr Med Res Opin 36(12):2009–2018
Emond B, Sundaram M, Romdhani H, Lefebvre P, Wang S, Mato A (2019) Comparison of time to next treatment, health care resource utilization, and costs in patients with chronic lymphocytic leukemia initiated on front-line ibrutinib or chemoimmunotherapy. Clin Lymphoma Myeloma Leuk 19(12):763–775.e2
Sathyanarayanan V, Flowers CR, Iyer SP (2020) Comparison of access to novel drugs for lymphoma and chronic lymphocytic leukemia between India and the United States. JCO Glob Oncol 6:1124–1133
Mikudina B, Goodall M, Adler AI (2017) NICE guidance on ibrutinib for previously treated chronic lymphocytic leukaemia and untreated chronic lymphocytic leukaemia in the presence of 17p deletion or TP53 mutation. Lancet Oncol 18(3):289–290
Barnes JI, Divi V, Begaye A, Wong R, Coutre S, Owens DK et al (2018) Cost-effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia in older adults without deletion 17p. Blood Adv 2(15):1946–1956
Sinha R, Redekop WK (2018) Cost-effectiveness of ibrutinib compared with obinutuzumab with chlorambucil in untreated chronic lymphocytic leukemia patients with comorbidities in the United Kingdom. Clin Lymphoma Myeloma Leuk 18(2):e131–e142
Alrawashdh N, McBride A, Erstad B, Sweasy J, Persky DO, Abraham I (2022) Cost-effectiveness and economic burden analyses on all first-line treatments of chronic lymphocytic leukemia. Value Health 25(10):1685–1695
Visentin A, Mauro FR, Catania G, Fresa A, Vitale C, Sanna A et al (2022) Obinutuzumab plus chlorambucil versus ibrutinib in previously untreated chronic lymphocytic leukemia patients without TP53 disruptions: a real-life CLL campus study. Front Oncol 21(12):1033413. https://doi.org/10.3389/fonc.2022.1033413 PMID: 36479077; PMCID: PMC9719965
Nehra P, Chauhan AS, Malhotra P, Kumar L, Singh A, Gupta N, et al (2023) Cost-effectiveness analysis of different combination therapies for the treatment of chronic lymphocytic leukaemia in India. Lancet Reg Health Southeast Asia 13,100201 [Accessed on 20-07-2023 from. https://www.thelancet.com/journals/lansea/article/PIIS2772-3682(23)00061-6/fulltext]
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hegde, N.C., Kumar, A., Kaundal, S. et al. Generic ibrutinib a potential cost-effective strategy for the first-line treatment of chronic lymphocytic leukaemia. Ann Hematol 102, 3125–3132 (2023). https://doi.org/10.1007/s00277-023-05342-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05342-y