Abstract
Objective
Our objective was to estimate the cost effectiveness of ofatumumab plus chlorambucil (OChl) versus chlorambucil in patients with chronic lymphocytic leukaemia for whom fludarabine-based therapies are considered inappropriate from the perspective of the publicly funded healthcare system in Canada.
Methods
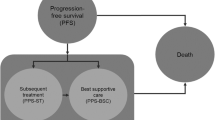
A semi-Markov model (3-month cycle length) used survival curves to govern progression-free survival (PFS) and overall survival (OS). Efficacy and safety data and health-state utility values were estimated from the COMPLEMENT-1 trial. Post-progression treatment patterns were based on clinical guidelines, Canadian treatment practices and published literature. Total and incremental expected lifetime costs (in Canadian dollars [$Can], year 2013 values), life-years and quality-adjusted life-years (QALYs) were computed. Uncertainty was assessed via deterministic and probabilistic sensitivity analyses.
Results
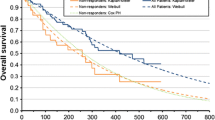
The discounted lifetime health and economic outcomes estimated by the model showed that, compared with chlorambucil, first-line treatment with OChl led to an increase in QALYs (0.41) and total costs ($Can27,866) and to an incremental cost-effectiveness ratio (ICER) of $Can68,647 per QALY gained. In deterministic sensitivity analyses, the ICER was most sensitive to the modelling time horizon and to the extrapolation of OS treatment effects beyond the trial duration. In probabilistic sensitivity analysis, the probability of cost effectiveness at a willingness-to-pay threshold of $Can100,000 per QALY gained was 59 %.
Conclusions
Base-case results indicated that improved overall response and PFS for OChl compared with chlorambucil translated to improved quality-adjusted life expectancy. Sensitivity analysis suggested that OChl is likely to be cost effective subject to uncertainty associated with the presence of any long-term OS benefit and the model time horizon.





Similar content being viewed by others
References
Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER cancer statistics review, 1975–2010. In: National Cancer Institute. 2013. http://seer.cancer.gov/csr/1975_2010/. Accessed 27 Feb 2014.
Orphanet: prevalence of rare diseases: bibliographic data. Paris: Orphanet; 2011.
Seftel MD, Demers AA, Banerji V, Gibson SB, Morales C, Musto G, Pitz MW, Johnston JB. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33:1463–8.
Public Health Agency of Canada. Chronic lymphocytic leukemia incidence [age-adjusted, 2009]. In: Chronic Disease Infobase. 2014. http://66.240.150.17:9600/PHAC/dimensionMembers.jsp?l=en-US,en;q=0.8&rep=iC3EE3A042BDA4B10ACDD99A9BB6ED430. Accessed 6 Mar 2014.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–56.
Eichhorst B, Hallek M, Dreyling M, ESMO Guidelines Working Group. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnostic, treatment, and follow-up. Ann Oncol. 2010;21(suppl 5):162–4.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grünhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jäger U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Bühler A, Winkler D, Zenz T, Böttcher S, Ritgen M, Mendila M, Kneba M, Döhner H, Stilgenbauer S, International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74.
Del Giudice I, Mauro FR, Foa R. Chronic lymphocytic leukemia in less fit patients: “slow-go”. Leuk Lymphoma. 2011;52:2207–16.
Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M, Kranzhöfer N, Rohrberg R, Söling U, Burkhard O, Westermann A, Goede V, Schweighofer CD, Fischer K, Fink AM, Wendtner CM, Brittinger G, Döhner H, Emmerich B, Hallek M, German CLL, Study Group (GCLLSG). First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–91.
Alberta Health Services. Chronic lymphocytic leukemia: clinical practice guideline LYHE-007 (version 3). 2015. http://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe007-cll.pdf. Accessed 13 Aug 2015.
Woyach JA, Ruppert AS, Rai K, Lin TS, Geyer S, Kolitz J, Appelbaum FR, Tallman MS, Belch AR, Morrison VA, Larson RA, Byrd JC. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential cancer and leukemia group B studies. J Clin Oncol. 2012;31:440–7.
Pan-Canadian Oncology Drug Review. Final recommendation for bendamustine (Treanda) for first line CLL. In: pCODR Expert Review Committee (pERC) final recommendation. 2013. http://www.pcodr.ca/idc/groups/pcodr/documents/pcodrdocument/pcodr-treandacll1st-fn-rec.pdf. Accessed 7 Aug 2015.
Pan-Canadian Oncology Drug Review. Bendamustine (Treanda) for CLL (first line). In: Provincial Funding Summary. 2013. http://www.pcodr.ca/idc/groups/pcodr/documents/webcontent/pcodr_provfund_treandacll1stln.pdf. Accessed 7 Aug 2015.
Arzerra (ofatumumab) [product monograph]. Mississauga (Ontario): GlaxoSmithKline Inc; 2014.
Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, Doubek M, Panagiotidis P, Kimby E, Schuh A, Pettitt AR, Boyd T, Montillo M, Gupta IV, Wright O, Dixon I, Carey JL, Chang CN, Lisby S, McKeown A, Offner F, COMPLEMENT 1 Study Investigators. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385(9980):1873–83.
GlaxoSmithKline. A phase III, open label, randomized, multicenter trial of ofatumumab added to chlorambucil versus chlorambucil monotherapy in previously untreated patients with chronic lymphocytic leukemia. 2013. http://www.gsk-clinicalstudyregister.com/study/OMB110911#rs. Accessed 29 Jul 2015.
Caro JJ, Briggs AH, Siebert U, Kuntz KM, ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices-overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15:796–803.
Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Med Decis Making. 2012;32(5):690–700.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006.
Mittmann N, Evans WK, Rocchi A, Longo CJ, Au H-J, Husereau D, Leighl N, Isogai P, Krahn M, Peacock S, Marshall D, Coyle D, Malfair Taylor SC, Jacobs P, Oh PI. Addendum to CADTH’s guidelines for the economic evaluation of health technologies: specific guidance for oncology products. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health. 2012;15(6):843–50.
Woods B, Hawkins N, Dunlop W, O’Toole A, Bramham-Jones S. Bendamustine versus chlorambucil for the first-line treatment of chronic lymphocytic leukemia in England and Wales: a cost-utility analysis. Value Health. 2012;15(5):759–70.
National Institute for Health and Care Excellence. Bendamustine for the first-line treatment of chronic lymphocytic leukaemia. In: NICE technology appraisal guidance 216. 2011. http://www.nice.org.uk/guidance/ta216/chapter/1-guidance. Accessed 7 Aug 2015.
Scottish Medicine Consortium. Bendamustine hydrochloride 25 mg, 100 mg powder for solution for infusion (Levact). In: SMC No. 694/11. 2011. http://www.scottishmedicines.org.uk/files/advice/bendamustine_Levact_CLL_FINAL_MARCH_2011_for_website.pdf. Accessed 7 Aug 2015.
Robak T, Dmoszynska A, Solal-Céligny P, Warzocha K, Loscertales J, Catalano J, Afanasiev BV, Larratt L, Geisler CH, Montillo M, Zyuzgin I, Ganly PS, Dartigeas C, Rosta A, Maurer J, Mendila M, Saville MW, Valente N, Wenger MK, Moiseev SI. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–65.
Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, Böttcher S, Staib P, Kiehl M, Eckart MJ, Kranz G, Goede V, Elter T, Bühler A, Winkler D, Kneba M, Döhner H, Eichhorst BF, Hallek M, Wendtner CM. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29(26):3559–66.
Raphael B, Andersen JW, Silber R, Oken M, Moore D, Bennett J, Bonner H, Hahn R, Knospe WH, Mazza J, et al. Comparison of chlorambucil and prednisone versus cyclophosphamide, vincristine, and prednisone as initial treatment for chronic lymphocytic leukemia: long-term follow-up of an Eastern Cooperative Oncology Group randomized clinical trial. J Clin Oncol. 1991;9:770–6.
Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, Juliusson G, Postner G, Gercheva L, Goranov S, Becker M, Fricke HJ, Huguet F, Del Giudice I, Klein P, Tremmel L, Merkle K, Montillo M. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84.
Goede V, Fischer K, Humphrey K, Asikanius E, Busch R, Engelke A, Wendtner VM, Samoylova O, Chagorova T, Dilhuydy M-S, De La Serna Torroba J, Illmer T, Opat S, Owen C, Kreuzer KA, Langerak AW, Ritgen M, Stilgenbauer S, Wenger M, Hallek M, German CLL Study Group. Obinutuzumab (GA101) plus chlorambucil (Clb) or rituximab (R) plus Clb versus Clb alone in patients with chronic lymphocytic leukemia (CLL) and preexisting medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase III trial. Abstract presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31–June 4 2013b. Abstract No. 7004.
Rai KR, Peterson BL, Appelbaum FR, Tallman MS, Belch A, Morrison VA, Larson RA. Long-term survival analysis of the North American Intergroup Study C9011 comparing fludarabine (F) and chlorambucil (C) in previously untreated patients with chronic lymphocytic leukemia (CLL). Abstract presented at the 51st Annual Meeting and Exposition of the American Society of Hematology, New Orleans, LA, 5–8 December 2009. https://ash.confex.com/ash/2009/webprogram/Paper20588.html. Accessed 7 Aug 2015.
Barendregt JJ. The half-cycle correction: banish it rather than explain it. Med Decic Mak. 2009;29(4):500–2.
Hawe E, Pearson I, Wolowacz S, Haiderali A. Use of external data to guide long-term survival extrapolations of trial data for chronic lymphocytic leukemia. 17th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research, Amsterdam, The Netherlands, 8–12 November 2014.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Briggs A, Claxton K, Sculpher M, editors. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Statistics Canada. Table 326-0021. Consumer Price Index (CPI), 2011 basket, annual (2002 = 100 unless otherwise noted), CANSIM (database). January 23, 2014. http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=3260021&paSer=&pattern=&stByVal=1&p1=1&p2=37&tabMode=dataTable&csid. Accessed 18 Nov 2014.
Ontario Drug Benefit. 2014. https://www.healthinfo.moh.gov.on.ca/formulary/index.jsp. Accessed 17 Jan 2014.
Tam VC, Ko YJ, Mittmann N, Cheung MC, Kumar K, Hassan S, Chan KK. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20:e90–106.
Ontario Ministry of Health and Long-term Care. 2014. http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx. Accessed 30 Jan 2014.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Can. 2006;95(6):683–90.
Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, Bramham-Jones S. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50.
IMS Brogan. Delta PA online database. 2013. https://baeappsw.imshealth.com/IMAC/Dashboard.aspx?Module=DeltaPA. Accessed 27 Jan 2014.
Ferguson J, Tolley K, Gilmour L, Priaulx J. Health state preference study mapping the change over the course of the disease process in chronic lymphocytic leukemia (CLL). Abstract No. PCN79. Abstract presented at the 11th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research, Philadelphia, PA, 20–24 November 2008.
Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8(1):1–2.
Statistics Canada. Table 281-0027. Earnings, average weekly, by province and territory. CANSIM. 2013. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/labr79-eng.htm. Accessed 11 Dec 2013.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programs. 3rd ed. Oxford: Oxford University Press; 2005.
Marsh K, Xu P, Orfanos P, Gordon J, Griebsch I. Model-based cost-effectiveness analyses for the treatment of chronic lymphocytic leukaemia: a review of methods to model disease outcomes and estimate utility. Pharmacoeconomics. 2014;32(10):981–93.
Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7(2):e31115.
Szabo SM, Levy AR, Davis C, Holyoake TL, Cortes J. A multinational study of health state preference values associated with chronic myelogenous leukemia. Value Health. 2010;13(1):103–11.
Acknowledgments
The authors would like to acknowledge the contribution of RTI Health Solutions employees Santiago Zuluaga-Sanchez, James Brockbank and Christopher Graham for technical writing assistance; Emma Hawe and Adam Irving for statistical analyses of COMPLEMENT-1 (survival analyses, treatment consumption, and design of EQ-5D analyses) and overall survival extrapolation guided by external data; and Sarah Mitchell, Matthew Woods and James Brockbank for performing the clinical and economic systematic literature reviews.
The authors also would like to acknowledge the clinical input received from the following: Dr James Kaye of RTI Health Solutions; all of the UK and Canadian clinical and health economics experts who shared their expertise on CLL and economic modelling; Tingting Song of Pharmaceutical Product Development for the utility score regression analysis; and Thomas Delea and Alice Wang of Policy Analysis Inc. for the physician survey analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding for this study was provided to RTI Health Solutions under a research contract with GlaxoSmithKline. Additional funding for the manuscript preparation and revision was provided to RTI Health Solutions under a research contract with Novartis Pharmaceuticals Canada Inc. During the conduct of the study, Dr. Hamid Reza Nakhaipour and Dr. Amin Haiderali were employees of GlaxoSmithKline. Dr. Nakhaipour is currently an employee of Astellas Pharma Inc. Dr. Haiderali is currently an employee of Merck & Co. Inc. Kavisha Jayasundara is an employee of GlaxoSmithKline. Dr. William Herring, Dr. Molly Purser, Dr. Isobel Pearson and Dr. Sorrel Wolowacz are employees of RTI Health Solutions, an independent research organization, and contributed to the design and development of the model, including input parameter identification, data analysis and interpretation of results. All authors participated in the conceptualization of the model and in the preparation of the manuscript. Additionally, Dr. Nakhaipour and Ms Jayasundara contributed to the identification of Canadian-specific input parameters, including the development of the survey of Canadian physicians. Dr. Herring, Dr. Purser, Dr. Pearson and Dr. Wolowacz contributed to the identification of input parameters, the model programming, and the interpretation of analytical results. Dr. Herring will serve as the overall guarantor for the content of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herring, W., Pearson, I., Purser, M. et al. Cost Effectiveness of Ofatumumab Plus Chlorambucil in First-Line Chronic Lymphocytic Leukaemia in Canada. PharmacoEconomics 34, 77–90 (2016). https://doi.org/10.1007/s40273-015-0332-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0332-5