Abstract
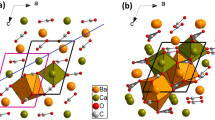
The temperature (T) evolution of the barium carbonate (BaCO3) structure was studied using Rietveld structure refinements based on synchrotron X-ray diffraction and a powdered synthetic sample. BaCO3 transforms from an orthorhombic, Pmcn, α phase to a trigonal, R3m, β phase at 811°C. The orthorhombic BaCO3 structure is isotypic with aragonite, CaCO3. In trigonal R3m BaCO3, the CO3 group occupies one orientation and shows no rotational disorder. The average <Ba–O> distances increase while the <C–O> distances decrease linearly with T in the orthorhombic phase. After the 811°C phase transition, the <Ba–O> distances increase while C–O distances decrease. There is also a significant volume change of 2.8% at the phase transition.







Similar content being viewed by others
References
Antao SM, Mulder WH, Hassan I, Crichton W, Parise JB (2004) Cation disorder in dolomite, CaMg(CO3)2, and its influence on the aragonite + magnesite → dolomite reaction boundary. Am Mineral 89:1142–1147
Baker EH (1962) High-temperature form of strontium carbonate. J Chem Soc 1962:2525–2526
Berg GW (1986) Evidence for carbonate in the mantle. Nature 324:50–51
Bevan DJM, Rossmanith E, Mylrea DK, Ness SE, Taylor MR, Cuff C (2002) On the structure of aragonite—Lawrence Bragg revisited. Acta Crystallogr B 58:448–456
Boeke HE (1913) The fusion of carbonates under carbon dioxide pressure. Mitt Naturforsch Ges Halle 3:1–12
Carlson WD (1983) The polymorphs of CaCO3 and the aragonite–calcite transformations. Mineral Soc Am Rev Mineral 11:191–225
De Villiers JPR (1971) Crystal structures of aragonite, strontianite, and witherite. Am Mineral 56:758–767
Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Hausermann D (1996) Two-dimensional detector software: from real detector to idealised image to two-theta scan. High Press Res 14:235–248
Hodgman CD (1955) Handbook of chemistry and physics. Chemical Rubber Publishing Co, Cleveland, p 479
Holl CM, Smyth JR, Laustsen HMS, Jacobsen SD, Downs RT (2000) Compression of witherite to 8 GPa and the crystal structure of BaCO3II. Phys Chem Miner 27:467–473
Lander JJ (1949) Polymorphism and anion rotational disorder in the alkaline earth carbonates. J Chem Phys 17:892–901
Larson AC, Von Dreele RB (2000) General Structure Analysis System (GSAS). Los Alamos National Laboratory Report, LAUR 86–748
Lin CC, Liu LG (1997) High pressure phase transformations in aragonite-type carbonates. Phys Chem Miner 24:149–157
Markgraf SA, Reeder RJ (1985) High-temperature structure refinements of calcite and magnesite. Am Mineral 70:590–600
Megaw HD (1973) Crystal structures: a working approach. W Saunders, Philadelphia
Ono S (2007) New high-pressure phases in BaCO3. Phys Chem Miner. Online doi:10.1007/s00269-006-0140-8
Ono S, Kikegawa T, Ohishi Y, Tsuchiya J (2005) Post-aragonite phase transformation in CaCO3 at 40 GPa. Am Mineral 90:667–671
Santillan J, Williams Q (2004) A high pressure X-ray diffraction study of aragonite and the post-aragonite phase transition in CaCO3. Am Mineral 89:1348–1352
Speer JA (1983) Crystal chemistry and phase relations of orthorhombic carbonates. Mineral Soc Am Rev Mineral 11:145–189
Strømme KO (1969) The crystal structure of sodium nitrate in the high-temperature phase. Acta Chem Scand 23:1616–1624
Strømme KO (1969) On the crystal structure of potassium nitrate in the high temperature phases I and III. Acta Chem Scand 23:1625–1636
Strømme KO (1972) On the mechanism of the continuous transformation in sodium nitrate. Acta Chem Scand 26:477–482
Strømme KO (1975) Crystal-structures of high-temperature forms of strontium and barium carbonate and structurally related compounds. Acta Chem Scand A 29:105–110
Toby BR (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34:210–221
Acknowledgments
This research was carried out in part at the National Synchrotron Light Source, Brookhaven National Laboratory (BNL), which is supported by the U.S. Department of Energy, Division of Materials Sciences and Division of Chemical Sciences, under Contract No. DE-AC02-98CH10886. We thank J. C. Hanson for his help in performing the synchrotron experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antao, S.M., Hassan, I. BaCO3: high-temperature crystal structures and the Pmcn→R3m phase transition at 811°C. Phys Chem Minerals 34, 573–580 (2007). https://doi.org/10.1007/s00269-007-0172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-007-0172-8