Abstract
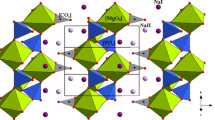
Single crystals of norsethite-type carbonate BaMn(CO3)2 up to 200 μm in size were synthesized in a closed cavity under high pressure–temperature (P–T) conditions. Electron microprobe analyses revealed the composition of 49.00–49.09 wt% BaO and 22.66–22.74 wt% MnO, which correspond well to the ideal formula of Ba1.0Mn1.0(CO3)2. Accurate crystalline structural data were determined from single crystal X-ray diffraction (XRD). The \(R\overline{3} c\) space-group with a doubled c-axis and \(R\overline{3} m\) space-group were used to refine the crystal structure of BaMn(CO3)2. It is proved that \(R\overline{3} m\) is the most probable space-group for the BaMn(CO3)2 crystal structure because no superstructure reflections were observed in the X-ray images. The unit cell parameters were identified to be a = 5.0827(2) Å and c = 17.2797(10) Å in the rhombohedral symmetry of the \(R\overline{3} m\) space-group with a final R-value of 0.0184. High-pressure Raman spectroscopy was performed up to 10 GPa at room temperature, and Raman band shifts (\(\frac{{{\text{d}}\nu_{i} }}{{{\text{d}}P}}\)) were quantified. Each Raman vibration underwent resolvable splitting and the corresponding \(\frac{{{\text{d}}\nu_{i} }}{{{\text{d}}P}}\) showed a pronounced jump as the pressure reached 3.8 GPa arising from a pressure-induced transition.






Similar content being viewed by others
References
Bischoff WD, Sharma SK, MacKenzie FT (1985) Carbonate ion disorder in synthetic and biogenic magnesian calcites: a Raman spectral study. Am Miner 70:581–589
Böttcher ME (2000) Stable isotope fractionation during experimental formation of norsethite (BaMg[CO3]2): a mineral analogue of dolomite. Aquat Geochem 6:201–213
Böttcher ME, Effenberger HS, Gehlken P-L, Grathoff GH, Schmidt BC, Geprägs P, Bahlo R, Dellwig O, Leipe T, Winde V, Deutschmann A, Stark A, Gallego-Torres D, Martinez-Ruiz F (2012) BaMn[CO3]2—a previously unrecognized double carbonate in low-temperature environments: structural, spectroscopic, and textural tools for future identification. Chem Erde 72:85–89
Chang LLY (1964) Synthesis of MBa(CO3)2 compounds. Am Miner 49:1142–1143
Damyanov ZK, Bakardjiev S, Popov P (1996) Mineralogy, geology and genesis of the Kremikovtsi carbonate-hosted submarine exhalative iron(+Mn)–barite (+base metals) deposit, West Balkan, Bulgaria. In: Proceedings of the Ann. Meet., Sofia, 1996, UNESCO Project 356, vol 1, pp 29–37
Effenberger H, Zemann J (1985) Single crystal X-ray investigation of norsethite, BaMg(CO3)2: one more mineral with an aplanar carbonate group. Zeitschrift fu¨r Kristallographie 171:275–280
Effenberger H, Pippinger T, Libowitzky E, Lengauer CL, Miletich R (2014) Synthetic norsethite, BaMg(CO3)2: revised crystal structure, thermal behaviour and displacive phase transition. Mineral Mag 78(7):1589–1612
Hood WC, Steidl PF, Tschopp DG (1974) Precipitation of norsethite at room temperature. Am Miner 59:471–474
Kapustin JL (1965) Norsethite—the first find in USSR. Doklady Acad Nauk USSR 161:922–924 (in Russian)
Lepland A, Stevens RL (1998) Manganese authigenesis in the landsort deep, Baltic Sea. Mar Geol 151:1–25
Lepland A, Sætherc O, Thorsnes T (2000) Accumulation of barium in recent Skagerrak sediments: sources and distribution controls. Mar Geol 163:13–26
Liang W, Yin Y, Li Z, Li R, Li L, He Y, Dong H, Li Z, Yan S, Zhai S, Li H (2018) Single crystal growth, crystalline structure investigation and high-pressure behavior of impurity-free siderite (FeCO3). Phys Chem Miner 45(9):831–842
Lindner M, Jordan G (2018) On the growth of witherite and its replacement by the Mg-bearing double carbonate norsethite: implications for the dolomite problem. Am Miner 103:252–259
Lindner M, Saldi GD, Jordan G, Schott J (2017) On the effect of aqueous barium on magnesite growth—a new route for the precipitation of the ordered anhydrous Mg-bearing double carbonate norsethite. Chem Geol 460:93–105
Lindner M, Saldi GD, Carrocci S, Be´ne´zeth P, Schott J, Jordan G (2018) On the growth of anhydrous Mg-bearing carbonates—implications from norsethite growth kinetics. Geochim Cosmochim Acta 238:424–437
Lippmann F (1968) Die Kristallstruktur von Norsethit. Tschermaks Mineral Petrogr Mitt 12:299–318
Mrose ME, Chao ETC, Fahey JJ, Milton C (1961) Norsethite, BaMg(CO3)2, a new mineral from the Green River formation, Wyoming. Am Miner 46:420–429
Onac BP (2002) Caves formed within Upper Cretaceous skarns at Băiţa, Bihor County, Romania: mineral deposition and speleogenesis. Can Mineral 40:1693–1703
Peacor DR, Essene EJ, Gaines AM (1987) Petrologic and crystal-chemical implications of cation order-disorder in kutnahorite CaMn(CO3)2. Am Miner 72:319–328
Pippinger T, Miletich R, Effenberger H, Hofer G, Lotti P, Merlini M (2014) High-pressure polymorphism and structural transitions of norsethite, BaMg(CO3)2. Phys Chem Miner 41:737–755
Platt RG, Woolley AR (1990) The carbonatites and fenites of Chipman lake, Ontario. Can Mineral 28:241–250
Reeder RJ (1983) Crystal chemistry of the rhombohedral carbonates. In: Reeder RJ (ed) Carbonates: mineralogy and chemistry. Reviews in mineralogy, vol 11. Mineralogical Society of America, Washington, pp 1–48
Reeder RJ, Dollase WA (1989) Structural variation in the dolomite-ankerite solid-solution series: an X-ray, Moessbauer, and TEM study. Am Miner 74:1159–1167
Ross NL, Reeder RJ (1992) High-pressure structural study of dolomite and ankerite. Am Miner 77:412–421
Schmidt B, Gehlken PL, Böttcher ME (2013) Vibrational spectra of BaMn(CO3)2 and a re-analysis of the Raman spectrum of BaMg(CO3)2. Eur J Mineral 25:137–144
Secco L, Lavina B (1999) Crystal chemistry of two natural magmatic norsethites, BaMg(CO3)2, from an Mg-carbonatite of the alkaline carbonatitic complex of Tapira (SE Brazil). Neues Jahrb Mineral Monatsh 1999:87–96
Sheldrick GM (2008) A short history of SHELX. Acta Cryst. A 2008(64):112–122
Steyn JGD, Watson MD (1967) A new occurrence of norsethite, BaMg(CO3)2. Am Miner 52:1770–1775
Sundius N, Blix R (1965) Norsethite from Langban. Arkiv Mineral Geol 4:277–278
White WB (1974) The carbonate minerals. In: Farmer VC (ed) The infra-red spectra of minerals, Mineralogical Society monograph, vol 4. Mineralogical Society, London, pp 227–284
Zheng YF, Böttcher ME (2014) Oxygen isotope fractionation in double carbonates. Isot Environ Health Stud 52:29–46
Zidarov N, Petrov O, Tarassov M, Damyanov Z, Tarassova E, Petkova V, Kalvachev Y, Zlatev Z (2009) Mn-rich norsethite from the Kremikovtsi ore deposit, Bulgaria. N Jb Mineral Abh 186:321–331
Acknowledgements
We appreciate Herta Effenberger and other two anonymous reviewers for their valuable comments and suggestions, which are important to greatly improve the manuscript quality. We also acknowledge Jung-Fu Lin from University of Texas at Austin for constructive discussion in carbonates minerals. This work was financially supported by Major State Research Development Program of China (2016YFC0601101), the National Science Foundation for Young Scientists of China (41802044), the Strategic Priority Research Program (B) of Chinese Academy of Sciences (XDB 18010401), 135 Program of the Institute of Geochemistry (Y2ZZ041000), CAS, and the Western Light (Y8CR028).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, W., Li, L., Yin, Y. et al. Crystal structure of norsethite-type BaMn(CO3)2 and its pressure-induced transition investigated by Raman spectroscopy. Phys Chem Minerals 46, 771–781 (2019). https://doi.org/10.1007/s00269-019-01038-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-019-01038-w