Abstract
Since the mid-1990s the surgical community has seen a surge in the prevalence of open abdomens (OAs) reported in the surgical literature and in clinical practice. The OA has proven to be effective in decreasing mortality and immediate postoperative complications; however, it may come at the cost of delayed morbidity and the need for further surgical procedures. Indications for leaving the abdomen open have broadened to include damage control surgery, abdominal compartment syndrome, and abdominal sepsis. The surgical options for management of the OA are now more diverse and sophisticated, but there is a lack of prospective randomized controlled trials demonstrating the superiority of any particular method. Additionally, critical care strategies for optimization of the patient with an OA are still being developed. Review of the literature suggests a bimodal distribution of primary closure rates, with early closure dependent on postoperative intensive care management and delayed closure more affected by the choice of the temporary abdominal closure technique. Invariably, a small fraction of patients requiring OA management fail to have primary fascial closure and require some form of biologic fascial bridge with delayed ventral hernia repair in the future.
Similar content being viewed by others
Introduction
Direct pressure has long been used as a means of hemostasis, and its application to abdominal hemorrhage, especially in the face of medical coagulopathy seems logical. In a seminal paper, Stone et al. wrote that abdominal tamponade with laparotomy sponges was a well known technique at the time to control solid organ injury. They described several patients transferred to Grady Hospital from referring physicians with “obvious packs protruding from the abdomen” [1]. Despite an improvement in survival from 7% to 65% in the original case series of Stone et al., historically, leaving the abdomen “open” was considered a surgical failure. Exiting the operative theater before completing all definitive repairs was thought to result in increased intraabdominal abscess, intestinal fistulas, evisceration, multiorgan dysfunction, and mortality.
In 1993, Rotondo et al. hypothesized that with new weaponry the injury patterns of trauma patients had changed and that although definitively addressing all injuries may have been preferable in the past it may no longer be possible in patients with multiorgan and severe vascular injuries [2]. Additionally, as our understanding of the bloody vicious triad of coagulopathy, acidosis, and hypothermia grew, the need for abbreviated surgery and rapid return to the intensive care unit (ICU) for aggressive resuscitation was emphasized. Lastly, abdominal compartment syndrome (ACS) was increasingly recognized as a contributing factor for mortality in these patients [3]. Faced with more severely injured patients and armed with an improved understanding of the pathophysiology of inflammation, injury response, and ACS, surgeons have reversed their original opinion of damage control surgery (DCS).
Damage control (DC) laparotomy and the open abdomen (OA) are now viewed as critical techniques in the treatment of severely injured patients. Additionally, DCS and use of the OA are being applied outside of the realm of trauma to patients with abdominal sepsis, prolonged or extensive elective surgery, and ACS (Table 1).
The increasing prevalence of the OA has prompted the development of numerous methods of temporary abdominal closure (TAC). The ideal TAC device contains the abdominal viscera during resuscitation and transport, limits contamination, prevents evisceration and desiccation, assists with evacuation of abdominal fluid, decreases bowel edema, prevents adhesions, allows easy access to the abdominal cavity, avoids damage to the fascial edges, and prevents abdominal wall retraction while allowing for expansion of abdominal contents to prevent the development of ACS [22–24].
Intensive care management of the OA is still in its infancy. Resuscitation should include not only aggressive correction of coagulopathy, acidosis, and hypothermia but may need to address paralysis, early enteral nutrition, and judicious fluid management. The optimal goal of early ICU management is to facilitate early closure (within the first 7 days) and prevent delayed complications, including failure of the primary fascial closure, enteroatmospheric fistulas, deep abdominal abscess, and massive ventral hernia.
Open abdomen
Definition
The open abdomen is defined simply as a surgical abdomen with the fascial edges purposefully left unapproximated. The OA is a planned surgical technique for managing DC trauma patients, severe abdominal sepsis, intraabdominal hypertension (IAH), necrotizing infections of the abdominal wall, and acute mesenteric ischemia [25]. It is a temporizing measure that allows a planned escape from the operating theater to control medical bleeding, correct metabolic derangements and hypothermia or facilitate repeated abdominal debridement or bowel resections.
Risk factors
Predictors of the need for DC procedures can be separated into preoperative and intraoperative factors. Ideally, the surgeon should already have decided upon definitive sugery or DCS upon entering the operating theater. Studies have found that an early decision to perform DCS may result in less mortality [26].
Preoperative predictors for DCS include penetrating torso trauma with hypotension, the need for resuscitative thoracotomy, blunt abdominal trauma with intraperitoneal hemorrhage and hypotension, severe pelvic fracture with hypotension, or severe multicavitary trauma [27]. Asensio et al. identified prehospital and emergency department (ED) variables that predicted exsanguinating hemorrhage and the need for DCS. Prehospital variables include an absence of pupillary response, spontaneous ventilation, extremity movements, sinus rhythm or carotid pulse, penetrating truncal trauma, and the need for intubation or cardiopulmonary resuscitation (CPR) in the field. ED variables included blood pressure <60 mmHg, inability to mount an appropriate tachycardic response, Revised Trauma Score <6, and admission pH < 7.2 [28]. Garrison et al. identified a high injury severity score, low Glasgow Coma Score (GCS), prolonged hypotension, hypothermia, and poor clotting function in the ED as predicting the need for DCS [29].
Early studies used intraoperative clinical triggers for DCS. Stone et al. used the surgeon’s recognition of the absence of clot formation and bleeding without a visible vascular source as a trigger for abbreviated laparotomy [1]. Rotondo et al. used abdominal exsanguination requiring 10 units of packed red blood cells (PRBC) to select patients for DCS [2]. Further experience with DCS has established the following risk factors for increased mortality and morbidity with definitive surgery: pH < 7.2, temperature <34°C, estimated blood loss (EBL) >4 l, transfusion >10 units PRBC, and systolic blood pressure <70 mmHg [28, 30]. Additionally, laboratory values including a base deficit >−6 in patients ≥55 years of age or >−15 in patients <55 years of age, lactate >5 mmol/l, prothrombin time >16, or partial thromboplastin time >50 have also been associated with the need for DCS and decreased survival [27, 31]. Any of these factors in a trauma patient undergoing open laparotomy should prompt the surgeon to perform an abbreviated first procedure and use TAC.
Damage control techniques and use of the OA may also be utilized in other populations. In fact, the treatment of ACS provides the only level I evidence in the literature supporting OA techniques [32]. Patients with ACS, defined as sustained intraabdominal pressure >20 mmHg with the onset of new organ dysfunction, should be managed with decompressive laparotomy and creation of an OA [33, 34]. Patients with acutely increased intraabdominal pressures >25 mmHg without acute organ dysfunction should also be considered for prophylactic decompressive laparotomy. Lastly, patients at high risk for postoperative ACS should be left open prophylactically following completion of surgery. These patients include those requiring >15 l of crystalloid, or 10 units of PRBC intraoperatively or patients with increased peak inspiratory pressures >40 mmHg upon fascial closure [3, 5, 28, 31, 32, 35].
The OA is also used in the management of “second-look” or staged laparotomy, which may occur after an embolic phenomenon or mesenteric venous occlusive disease. It is also employed in patients with severe abdominal sepsis necessitating repeated débridement, most specifically severe pancreatic necrosis. Planned relaparotomy versus demand laparotomy for abdominal sepsis have equivalent survivals with a tendency toward increased survival after planned relaparotomy [12, 36]. The most important factor determining which modality to choose intraoperatively is the ability to attain adequate initial source control [15]. Althugh no study to date has found a clear survival benefit between planned versus demand relaparotomy, van Ruler et al. did find a significant decrease in health care cost, reoperations, and length of ICU and hospital stays with demand laparotomy [37].
The use of DC techniques has been associated with less mortality, decreased multisystem organ dysfunction and infection, and decreased risk of ACS after trauma and emergency general surgery (Table 1). Once the goals of care have been changed to damage control, and the fascia is left open prophylactically or therapeutically, a decision as to the type of dressing or TAC must be made.
Temporary abdominal closure options
In the initial series of patients subjected to damage control, skin or even formal closure of the fascia was used to enhance the tamponade effect of packing. However, the resulting high incidence of ACS led to a significant decrease in the use of these types of closure [3, 8, 9]. Surgical techniques have subsequently been refined greatly during the past three decades, from the towel clip closure technique to the more sophisticated ABThera System (KCI, San Antonio, TX, USA). The evolution of TAC has focused on balancing the stabilization of the abdominal compartment with the surgeon’s need for swift, convenient application, removal, and replacement of the temporary system. The ideal TAC would contain intraabdominal viscera in a homeostatic environment while minimizing trauma to the skin, fascia, and underlying bowel. It would also limit contamination, provide a means of egress for peritoneal fluid, prevent adhesion formation and recurrent ACS, and be cost-effective. Arguably, the most important aspect of TAC would be to prevent retraction of the abdominal wall and facilitate future primary fascial closure [22]. The modern surgeon now has access to numerous TAC devices, both commercial and self-designed. Unfortunately, there is little evidence to favor one form of TAC over another, and well designed randomized controlled trials for trauma, emergency general surgery, and vascular patients are lacking (Table 2). The options can be divided into skin closure techniques, fascial closure techniques, and negative pressure applications. The most common of these are the Barker or other negative-pressure dressing, the Wittmann Patch, and the Bogota bag [32, 76].
Skin closure techniques
The skin closure technique uses skin to anchor the TAC and provide some abdominal wall stability with containment of the abdominal viscera. These techniques include simple running suture of the skin, sequential towel clip closure, the silo technique, and the Bogota bag. Each had its place in the early days of TAC.
Towel clip and suture closure of the skin is swift, inexpensive, and easily available. Both of the techniques, however, rely on a fixed circumference and bursting pressure of the skin; they therefore have an increased risk of evisceration, skin necrosis, infection, and recurrent ACS [77]. Towel clip closure creates a large radiopaque mass on the abdominal wall that limits the effectiveness of further radiologic studies. Because of the high complication rates, including that of ACS, varying from 13% to 36%, these techniques have largely been abandoned (Table 1).
The silo technique and Bogota bag (Fig. 1) require suturing an inert, nonpermeable barrier (sterile IV bag, bowel bag, Steri-Drape, Silastic cloth) to the skin or fascia to contain the abdominal viscera. The Bogota bag was invented by Oswaldo Borraez in 1984, but the term was coined by Mattox after visiting with surgeons in Bogota, Colombia in 1997 [78, 79]. These techniques are inexpensive, have swift application, can be used as a temporary measure, and allow some abdominal wall stabilization. However, both are prone to leakage and evisceration, do not prevent abdominal wall retraction, and do not allow effective removal of abdominal fluid [22, 77]. The rates of primary closure vary depending on patient population and range from 12 to 82%, primarily <30% (Table 2). The plastic or Silastic material does allow expansion of viscera outside the abdominal cavity, but it limits expansion to a fixed volume; and the subsequent incidence of ACS ranges from 2.3 to 33.0%, somewhat lower than those of the skin closure methods (Table 2). Enterocutaneous fistula rates vary but are generally low, ranging from 0 to 14.4% (Table 2).
Fascial closure techniques
The fascial closure techniques (FCTs) use an interposition graft material sutured to the abdominal fascia. These techniques can utilize absorbable materials such as Vicryl or biological mesh, and nonabsorbable grafts such as the Wittmann Patch, polypropylene (PPE) mesh, Esmark bandages, and expanded polytetrafluoroethylene (ePTFE). Initially, the graft material should be redundant so the TAC is “loose,” thereby allowing visceral swelling and thus preventing the development of ACS. As the visceral edema resolves, the TAC is sequentially tightened by excising the central portion of the graft and resuturing the material closed to facilitate fascial approximation during reexploration [50, 52, 53, 75]. Alternatively, as in the case of the Wittmann Patch, refastening and tightening the trademark hook system achieves fascial approximation. Typically, the mesh is sequentially tightened every 24–48 h until the fascia is approximately 2–4 cm apart, at which point the fascia is closed primarily [36, 57, 58].
These techniques provide a mechanism to limit or even reverse the loss of domain that occurs in the OA from lack of fascial tension. Because of this, FCTs should be considered when the OA is unlikely to be closed within the first week [57, 65, 69]. FCTs have extended the time for primary closure to >50 days by the use of progressive fascial tension [53]. FCT (specifically the Wittmann Patch) can facilitate reexploration. However, these techniques are more costly and require special equipment not readily available to all surgeons. In addition, FCTs do not prevent adhesions of the viscera to the anterior abdominal wall and therefore may limit the ability to mobilize the abdominal wall for primary closure. FCTs also require suturing to the abdominal fascia, which may increase the risk of fascial trauma and necrosis, and future incisional hernias may develop. Lastly, this technique does not provide a means to evacuate peritoneal fluid, and abdominal wound drainage may become an issue resulting in recurrent intraabdominal hypertension or maceration of the surrounding wound edges.
The most significant drawbacks of FCTs in their early use were the low rate of primary closure with absorbable material and the high rate of fistulas with nonabsorbable mesh. Early series using absorbable mesh reported low rates of primary closure (18–38%), with moderate to high fistula rates (7–26%) (Table 2). During this time period, absorbable mesh was not used to approximate the fascial edges serially but was designed to form a bed of granulation tissue for future skin grafting and planned ventral hernia. Use of nonabsorbable meshes subsequently improved the primary closure rates, which ranged from 33 to 89%, but the fistula rates remained high (6–18%) [32]. Some series reported fistula formation in as many as 75% of patients if a nonadherent barrier or omentum was not placed over the bowel for protection [80].
The one FCT method that still enjoys popularity and appears to result in overall good outcomes is the Wittmann Patch (WP). This mesh utilizes a biologically compatible artificial material that has hooks and eyes that adhere to each other, similar to Velcro. This material can be sutured to the fascial edges, either at the time of the first procedure or at subsequent reexplorations if an extended duration of OA is anticipated [54]. The rate of primary closure for the WP ranges from 78 to 100%. The rate of complications remains relatively low overall, and the fistula rate is 0–4.2% (average 2%) [81].
Negative-pressure applications
Negative-pressure applications (NPAs) originated with Barker’s group in Chattanooga during the mid-1990s. They coined the term “vacuum pack” (VP) in 1995 [82]. In the initial study, a three-layered technique was used. The inner layer that faced the viscera consisted of a fenestrated inert sheet (IV bag or ISO 1010 Drape) covering the entire viscera to the bilateral paracolic gutters to prevent adhesions of viscera to the overlying peritoneum. The middle layer was made of Kerlex, lap sponges, gauze, or blue towels designed to provide the suction media for the NPA. It is imperative the middle layer not contact the underlying viscera, as it would increase the risk of fistula formation. Drains were placed in this layer to apply the negative pressure. The outer layer consisted of a bioocclusive adhesive sheet (Ioban) that was secured laterally to the flank skin and provided enough integrity to the abdominal wall to allow turning and positioning the patient prone if necessary. This three-layered vacuum pack was able to maintain visceral containment, prevent desiccation, and allow continuous evacuation of peritoneal fluids [60, 82]. The authors achieved primary fascial closure in 68% of patients with a 5% incidence of fistula formation [23]. Several groups have reported success using similar systems with primary fascial closure rates ranging from 35 to 92% (usually >50%). Fistula rates ranged from 0 to 15% (average 5.7%) [81].
The NPAs have been expanded to include two subsequent versions from KCI: the V.A.C. Abdominal Dressing System and ABThera System. Most data have been derived from the Abdominal Dressing System, but the ABThera uses the same technique with improved refinements. The abdominal dressing consists of an inner plastic-encased sponge designed to be in direct contact with the viscera. The plastic interface protects the bowel, prevents adhesion formation, and is perforated to allow passage of fluids. The plastic encasement can be cut to cover the entire fascial defect. Next, a macroporous GranuFoam “black” sponge is then applied over the inner layer and is in contact with the fascia and subcutaneous tissue. Careful attention should be taken to avoid direct contact with the skin. This sponge can be held in place with skin staples to approximate the skin edges if desired. This layer is then covered with an adhesive occlusive dressing; and a suction drainage system is applied to the superficial foam layer for evacuation of peritoneal fluid. The V.A.C. machine can be programmed for continuous or intermittent suction at various pressures as desired by the physician. Alternatively, a perforated plastic drape can be placed directly on the viscera and the microporous “white” sponge placed on the plastic followed by traditional macroporous GranuFoam; or GranuFoam can be used for the entire dressing [64, 65, 69].
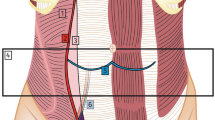
The newer ABThera system (Fig. 2) also has a visceral protective layer designed to cover the entire abdominal contents from pelvis to diaphragm to paracolic gutters, thereby preventing adhesions to the visceral mass and facilitating future abdominal wall mobilization. It has the added advantage of inner sponge extensions that extend to the ends of the plastic sheet to facilitate more effective evacuation of peritoneal fluid. This protected sponge can then be placed into the pelvis and deeply in the paracolic gutters to limit pooling in these dependent areas of the abdomen. Next, the GranuFoam, occlusive layer, and tubing set are applied as previously stated [83].
ABThera™ System by V.A.C. © has an inner layer that protects the abdominal viscera and facilitates evacuation of abdominal fluid and edema. The second layer has a macroporous GranuFoam that serves as the medium for direct application of negative pressure. The GranuFoam can be stapled to the skin to prevent the skin edges from rolling under and becoming macerated or ischemic from direct contact with the negative-pressure foam
These systems are designed to drain peritoneal effluent, minimize visceral edema, facilitate abdominal wall mobilization, and minimize the loss of domain. Some proponents suggest that the VAC system applies more fascial tension and prevents abdominal wall retraction better than the SCT and the Barker VP systems, however there are few data to support these claims [83]. Primary fascial closure rates utilizing these systems range from 33 to 100% (average 67%), similar to the vacuum pack. The fistula rates remain low, at 0–15% (average 2.9%) [81].
Review of studies involving NPA suggests that the risk of fistula is increased with intraabdominal sepsis and anastomoses directly underneath the vacuum pack, particularly colonic or duodenal anastomoses. Fistula rates were also increased by an increased duration of OA and in patients in whom primary closure was not possible [71]. In the NPA studies that found the highest primary fascial closure rates (>80%), the wound V.A.C. or Vac Pac was used in conjunction with some form of fascial tension technique. Suliburk et al. [64] and Miller et al. [65] used sequentially placed interrupted fascial sutures every 48 h in addition to the V.A.C. to achieve primary closure of 86%. Cothren and associates created abdominal wall tension with horizontal mattress sutures through the fascia in addition to the V.A.C. abdominal dressing. These sutures were sequentially tightened or replaced during subsequent operating room visits until primary fascial approximation was achieved. Although a time-consuming process, this technique achieved 100% fascial closure in 14 patients [69].
In many case series, an NPA is used following the first procedure, and FCT is utilized at the time of the first reexploration if closure is not anticipated in a timely fashion [22]. This sequence minimizes rates of ACS during the highest risk period of active resuscitation and assists in evacuating large volumes of peritoneal fluid. The FCT then improves the chances of primary closure by creating tension on the fascial edges during the subacute period.
There appear to be two groups of patients with OA. The first group is relatively uncomplicated and can be closed within 4–7 days. These patients generally have a high rate of primary fascial closure and likely do well regardless of the choice of TAC [23, 66, 67]. The second group, for myriad reasons, have more complicated and prolonged resuscitative efforts and hospital courses. The timing of abdominal closure in this group tends to extend beyond 7 days, generally to 20–40 days [53, 55, 57, 58, 65, 67, 69]. The rates of primary closure are much lower in these patients, and the type of TAC chosen may have a significant impact on their ability to achieve primary facial closure. Several risk factors have been found that predict a more prolonged or complicated course—and subsequently a diminished rate of primary closure: prolonged duration of OA; multisystem injuries, particularly colonic or duodenal; and active infection [22, 67, 71].
A study of OA in trauma patients revealed that surgical site and bloodstream infections resulted in a doubling of time to closure, and all infections resulted in decreased primary closure rates. In fact, simply having a white blood cell count >20,000 cells/ml was associated with delays in primary closure [84].
Several studies have also indicated that patients with conservative fluid resuscitation (<20 l) and fewer transfusions, or a net negative fluid balance, have improved rates of primary closure [66, 67, 71, 84]. A study by Stone et al. suggested that patients with OA and persistent tissue hypoperfusion and an inability to clear elevated lactate levels were also less likely to be closed primarily [66].
Management of patients with an open abdomen
The intensive care management of the OA is important to the surgical success of primary fascial closure. The early postoperative effort should be focused on correcting the oxygen and energy debt, hypothermia, and coagulopathy within the first 24 h [27]. Physicians should continue resuscitation with 1:1 replacement of blood products until the coagulation profile has corrected and bleeding has ceased before resuscitation with a mixture of crystalloid and colloid supplementation [85–87]. Historically, the surgical community has advocated aggressive and liberal crystalloid infusion to correct hemodynamic and metabolic derangements. However, this can lead to volume overload and increased risks of ACS, pulmonary edema, and acute respiratory distress syndrome. Although no randomized controlled trials of restrictive fluid administration and OA have been attempted, judicious intravenous fluid resuscitation targeting dynamic hemodynamic parameters (stroke volume variance or pulse pressure differential) versus static parameters (central venous pressure or left atrial pressure) may decrease the incidence of ACS and OA and increase early closure rates [88]. Our own institution’s anecdotal observation suggests that the current 1:1 blood product replacement strategy has greatly decreased the incidence and time requirement of the OA in the trauma patient population. Additionally, recent studies have found an association with restricted crystalloid fluid infusion (<20 l) or net negative fluid balance and improved primary closure rates [9, 89].
In patients with OA secondary to ACS or in those with large-volume resuscitation due to hemodynamic instability or prolonged bleeding, intraabdominal pressures should be monitored routinely as IAH or ACS can occur or recur [33, 34]. If bladder pressure exceeds 20 mmHg, the pressure should be monitored hourly; and if any evidence of organ dysfunction occurs, the TAC should be removed, and/or replaced with a larger dressing. Consideration may be given to extending the original incision as well [22].
Aside from resuscitation of the postsurgical OA, few data are available to make any firm recommendations. Questions remain regarding the need for neuromuscular blockade with a neuromuscular blocking agent (NMBA) and its timing and duration. Preliminary data suggest that enteral feeding is not harmful and may even be beneficial for an increased closure rate and decreased infection risk. Few to no data are available regarding antibiotic use, sedation level, pain management, use of diuretics, or ventilator management strategies.
Neuromuscular blockade
The use of neuromuscular blockade was proposed for the early treatment and management in IAH and ACS [33, 34]. De Laet et al. studied the effect of an NMBA in IAH prospectively using cisatracurium. Neuromuscular blockade did decrease intraabdominal pressure (IAP) (P < 0.002), however, once the paralysis wore off, the IAP returned to baseline levels. The study was not designed to determine long-term outcomes or prevention of progression to ACS [90]. It is possible to infer that continuous infusion of an NMBA may be beneficial for temporarily improving abdominal wall compliance and therefore facilitating primary fascial closure. A short course of an NMBA may decrease fascial edge retraction and be a useful adjunct to negative pressure devices and methods.
The only study directly assessing the OA and neuromuscular blockade analyzed 192 OA trauma patients who were divided into groups receiving a continuous infusion of an NMBA for >24 h versus patients not receiving an NMBA. The patients receiving the NMBA were more likely (93% vs. 83% P < 0.024) to have primary fascial closure by 7 days with no increase in rates of ventilator-associated pneumonia. In addition, multivariate regression analysis revealed neuromuscular blockade to have an odds ratio of 3.24 (P < 0.026) in favor of fascial closure, but there was no mention of the amount of perioperative fluid resuscitation, the blood transfusion requirement, the TAC technique, or the overall abdominal closure rate [91]. In addition, a retrospective study of 93 trauma patients with OA revealed that NMBA use was not a statistically significant predictor of primary closure [71]. Aside from these studies and anecdotal evidence, data are scarce regarding the use of NMBAs. Neuromuscular blockade historically has been considered relatively contraindicated in most patients because of its associated risks of increased ventilator-associated pneumonia, peripheral nerve injury, skin breakdown, and thromboembolic complications. Additionally, the use of NMBAs significantly increases the risks of ICU neuromyopathy. This is particularly true in patients who are receiving aminoglycosides or steroids, as is the case in many trauma or general surgical patients with OA who may have open fractures, gram-negative abdominal sepsis, or adrenal insufficiency [92].
Many of these complications occur primarily with prolonged NMBA use (>24–48 h). It may be reasonable to recommend a short course of continuous NMBA postoperatively while the patient is stabilized to facilitate primary fascial closure early. The potential increase in primary fascial closure rate with the associated decrease in OA complications should outweigh the risk of long-term NMBA sequela during its limited use. However, expert opinion on the routine use of NMBA in the ICU management of OA remains divided at this time.
Enteral feeding
The value of instituting early enteral feeding in the trauma or postoperative surgical patient has clearly been validated in numerous studies [93–95]. Patients with OA have traditionally been kept nil per os; however, Cheatham et al. challenged this when his group revealed that the OA results in significant protein loss (2 g/day) [96]. Subsequent work by Cothren et al. verified that tube feeds did not increase the risk of ACS in the recently closed OA [97]. Sufficient data now exist to suggest that patients with an OA clearly benefit from early enteral nutrition. Early feeds are associated with increased primary fascial closure and decreased intestinal fistula, infectious complications, ICU stays, and hospital costs [98–100].
Enteral access should be attained early via a nasogastric or nasojejunal feeding tube. Gastostrostomy or enteric tubes should be used with caution owing to the risk of leaks and fistulas as well as the potential compromise of future closure options [101]. Percutaneous enteral access is still possible in most patients once formal closure is attained [102].
Antibiotic use
There is evidence to support increasing or redosing prophylactic antibiotics intraoperatively if large-volume resuscitation is ongoing as significant fluid shifting may result in dilution of tissue and serum antibiotic concentrations [103–106]. There are no data to support continuation of antibiotic prophylaxis beyond 24 h in the case of trauma OA. Additionally, a study of OA in trauma patients found no correlation between the antibiotic type or its duration and primary closure rates [71]. If OA is utilized for abdominal sepsis, antibiotic coverage should initially be broad to cover the wide range of skin and bowel flora, and then tapered according to intraoperative culture results. Antibiotic duration should be determined by the length of treatment appropriate for the primary abdominal pathology (e.g., diverticulitis, pancreatitis, abscess) and discontinued when clinical signs of infection resolve. If no clinical evidence of infection is present and cultures show no growth, antibiotics should be discontinued [22].
Sedation/analgesia
There are no studies regarding optimal pain control or sedation in the patient with OA, but expert opinion suggests deep sedation may be of some benefit [22]. Epidural anesthesia has been shown to reduce intraabdominal pressures in ACS/IAH, but its application in the OA is unknown [107]. It is intuitive that deep sedation would decrease the force of abdominal wall retraction similar to or in conjunction with neuromuscular blockade.
Conclusions
The indications for the use of the open abdomen have expanded over the last two decades. Data in the form of prospective case series have shown decreased mortality and morbidity for trauma patients, those undergoing acute care surgery, and those with ACS. The OA should be used early in the management of these patients to gain the most benefit and prevent subsequent multiorgan failure.
Surgeons have various commercial and noncommercial options for managing the OA. The wound Vac and Vac Pac have clear advantages early, including quick application, evacuation of abdominal fluid, minimal risk of IAH, low fistula rates, and good early closure rates. Once the OA enters a “chronic stage” at around days 7–10, wound closure devices, such as the Wittmann Patch, which applies constant tension on the fascial edge, reduce loss of domain and are associated with the highest rates of delayed primary fascial closure. The highest closure rates are achieved during the first 7–10 days. Therefore, every attempt should be made to achieve closure within this window.
Immediate postoperative management should be directed at resuscitation and correction of coagulopathy and oxygen debt. The subsequent focus should be on measures aimed at early closure. Neuromuscular blockade for the first 24–48 h seems to have some role in facilitating early closure. Early enteral nutrition within the first 48–72 h has clear benefit in decreasing the negative nitrogen balance, decreasing bowel edema, and achieving primary fascial closure. Future studies must address limiting crystalloid usage during the initial operation/trauma resuscitation, the role and timing of diuresis, appropriate sedation level, optimal ventilator management, antibiotic usage, and the possible benefit of early continuous venovenous hemodialysis.
References
Stone HH, Strom PR, Mullins RJ (1983) Management of the major coagulopathy with onset during laparotomy. Ann Surg 197:532–535
Rotondo MF, Schwab CW, McGonigal MD et al (1993) ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 35:375–382
Raeburn CD, Moore EE, Biffl WL et al (2001) The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg 182:542–546
Ivatury RR, Porter JM, Simon RJ et al (1998) Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma 44:1016–1021
Offner PJ, de Souza AL, Moore EE et al (2001) Avoidance of abdominal compartment syndrome in damage-control laparotomy after trauma. Arch Surg 136:676–681
Nicholas JM, Rix EP, Easley KA et al (2003) Changing patterns in the management of penetrating abdominal trauma: the more things change, the more they stay the same. J Trauma 55:1095–1108
Asensio JA, Petrone P, Roldan G et al (2004) Has evolution in awareness of guidelines for institution of damage control improved outcome in the management of the posttraumatic open abdomen? Arch Surg 139:209–214
Balogh Z, McKinley BA, Cocanour CS et al (2002) Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg 184:538–543
Balogh Z, McKinley BA, Holcomb JB et al (2003) Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma 54:848–859
De Waele JJ, Hoste E, Blot SI et al (2005) Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 9:R452–R457
Cheatham ML, Safcsak K (2010) Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med 38:402–407
Garcia-Sabrido JL, Tallado JM, Christou NV et al (1988) Treatment of severe intra-abdominal sepsis and/or necrotic foci by an ‘open-abdomen’ approach: zipper and zipper-mesh techniques. Arch Surg 123:152–156
Ivatury RR, Nallathambi M, Rao PM et al (1989) Open management of the septic abdomen: therapeutic and prognostic considerations based on APACHE II. Crit Care Med 17:511–517
Tsiotos GG, Luque-de Leon E, Soreide JA et al (1998) Management of necrotizing pancreatitis by repeated operative necrosectomy using a zipper technique. Am J Surg 175:91–98
Ozguc H, Yilmazlar T, Gurluler E et al (2003) Staged abdominal repair in the treatment of intra-abdominal infection: analysis of 102 patients. J Gastrointest Surg 7:646–651
Adkins AL, Robbins J, Villalba M et al (2004) Open abdomen management of intra-abdominal sepsis. Am Surg 70:137–140
Radenkovic DV, Bajec DD, Tsiotos GG et al (2005) Planned staged reoperative necrosectomy using an abdominal zipper in the treatment of necrotizing pancreatitis. Surg Today 35:833–840
Besselink MG, de Bruijn MT, Rutten JP et al (2006) Surgical intervention in patients with necrotizing pancreatitis. Br J Surg 93:593–599
Oelschlager BK, Boyle EM Jr, Johansen K et al (1997) Delayed abdominal closure in the management of ruptured abdominal aortic aneurysms. Am J Surg 173:411–415
Rasmussen TE, Hallett JW Jr, Noel AA et al (2002) Early abdominal closure with mesh reduces multiple organ failure after ruptured abdominal aortic aneurysm repair: guidelines from a 10-year case-control study. J Vasc Surg 35:246–253
Djavani K, Wanhainen A, Bjorck M (2006) Intra-abdominal hypertension and abdominal compartment syndrome following surgery for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 31:581–584
Open Abdomen Advisory Panel, Campbell A, Chang M, Fabian T et al (2009) Management of the open abdomen: from initial operation to definitive closure. Am Surg 75:S1–S22
Barker DE, Green JM, Maxwell RA et al (2007) Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg 204:784–792
Aydin C, Aytekin FO, Yenisey C et al (2008) The effect of different temporary abdominal closure techniques on fascial wound healing and postoperative adhesions in experimental secondary peritonitis. Langenbecks Arch Surg 393:67–73
Schecter WP, Ivatury RR, Rotondo MF et al (2006) Open abdomen after trauma and abdominal sepsis: a strategy for management. J Am Coll Surg 203:390–396
Hirshberg A, Wall MJ Jr, Mattox KL (1994) Planned reoperation for trauma: a two year experience with 124 consecutive patients. J Trauma 37:365–369
Wyrzykowski AD, Feliciano DV (2008) Trauma damage control. In: Feliciano DV, Mattox KL, Moore EE (eds) Trauma, 6th edn. McGraw-Hill, New York, pp 851–870
Asensio JA, McDuffie L, Petrone P et al (2001) Reliable variables in the exsanguinated patient which indicate damage control and predict outcome. Am J Surg 182:743–751
Garrison JR, Richardson JD, Hilakos AS et al (1996) Predicting the need to pack early for severe intra-abdominal hemorrhage. J Trauma 40:923–927
Cosgriff N, Moore EE, Sauaia A et al (1997) Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidosis revisited. J Trauma 42:857–861
Sharp KW, Locicero RJ (1992) Abdominal packing for surgically uncontrollable hemorrhage. Ann Surg 215:467–474
Diaz JJ Jr, Cullinane DC, Dutton WD et al (2010) The management of the open abdomen in trauma and emergency general surgery. Part 1. Damage control. J Trauma 68:1425–1438
Malbrain ML, Cheatham ML, Kirkpatrick A et al (2006) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med 32:1722–1732
Cheatham ML, Malbrain ML, Kirkpatrick A et al (2007) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med 33:951–962
Johnson JW, Gracias VH, Schwab CW et al (2001) Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma 51:261–269
Wittmann DH, Aprahamian C, Bergstein JM (1990) Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg 14:218–226. doi:10.1007/BF01664876
Van Ruler O, Mahler CW, Boer KR et al (2007) Comparison of on-demand vs. planned relaparotomy strategy in patients with severe peritonitis: a randomized trial. JAMA 298:865–872
Smith PC, Tweddell JS, Bessey PQ (1992) Alternative approaches to abdominal wound closure in severely injured patients with massive visceral edema. J Trauma 32:16–20
Tremblay LN, Feliciano DV, Schmidt J et al (2001) Skin only or silo closure in the critically ill patient with an open abdomen. Am J Surg 182:670–675
Kirshtein B, Roy-Shapira A, Lantsberg L et al (2007) Use of the “Bogota bag” for temporary abdominal closure in patients with secondary peritonitis. Am Surg 73:249–252
Brox-Jimenez A, Ruiz-Luque V, Torres-Arcos C et al (2007) Experience with the Bogota bag technique for temporary abdominal closure. Cir Esp 82:150–154
Batacchi S, Matano S, Nella A et al (2009) Vacuum-assisted closure device enhances recovery of critically ill patients following emergency surgical procedures. Crit Care 13:R194
Howdieshell TR, Yeh KA, Hawkins ML et al (1995) Temporary abdominal wall closure in trauma patients: indications, technique, and results. World J Surg 19:154–158. doi:10.1007/BF00317004
Doyon A, Devroede G, Viens D et al (2001) A simple, inexpensive, life-saving way to perform iterative laparotomy in patients with severe intra-abdominal sepsis. Colorectal Dis 3:115–121
Foy HM, Nathens AB, Maser B et al (2003) Reinforced silicone elastomer sheeting, an improved method of temporary abdominal closure in damage control laparotomy. Am J Surg 185:498–501
Howdieshell TR, Proctor CD, Sternberg E et al (2004) Temporary abdominal closure followed by definitive abdominal wall reconstruction of the open abdomen. Am J Surg 188:301–306
Bee TK, Croce MA, Magnotti LJ et al (2008) Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma 65:337–342
Mayberry JC, Burgess EA, Goldman RK et al (2004) Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: the plain truth. J Trauma 57:157–162
Jernigan TW, Fabian TC, Croce MA et al (2003) Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg 238:349–355
Tons C, Schachtrupp A, Rau M et al (2000) Abdominal compartment syndrome: prevention and treatment. Chirurg 71:918–926
Sugrue M, Jones F, Janjua KJ et al (1998) Temporary abdominal closure: a prospective evaluation of its effects on renal and respiratory physiology. J Trauma 45:914–921
Vertrees A, Kellicut D, Ottman S et al (2006) Early definitive abdominal closure using serial closure technique on injured soldiers returning from Afghanistan and Iraq. J Am Coll Surg 202:762–772
Vertrees A, Greer L, Pickett C et al (2008) Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg 207:801–809
Wittmann DH (2000) Staged abdominal repair: development and current practice of an advanced operative technique for diffuse suppurative peritonitis. Acta Chir Austriaca 32:171–178
Hadeed JG, Staman GW, Sariol HS et al (2007) Delayed primary closure in damage control laparotomy: the value of the Wittmann Patch. Am Surg 73:10–12
Keramati M, Srivastava A, Sakabu S et al (2008) The Wittmann Patch is a temporary abdominal closure device after decompressive celiotomy for abdominal compartment syndrome following burn. Burns 34:493–497
Weinberg JA, George RL, Griffin RL et al (2008) Closing the open abdomen: improved success with Wittmann Patch staged abdominal closure. J Trauma 65:345–348
Tieu BH, Cho SD, Luem N et al (2008) The use of the Wittmann Patch facilitates a high rate of fascial closure in severely injured trauma patients and critically ill emergency surgery patients. J Trauma 65:865–870
Smith LA, Barker DE, Chase CW et al (1997) Vacuum pack technique of temporary abdominal closure: a four-year experience. Am Surg 63:1102–1107; discussion 1107–1108
Barker DE, Kaufman HJ, Smith LA et al (2000) Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma 48:201–206
Garner GB, Ware DN, Cocanour CS et al (2001) Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg 182:630–638
Miller PR, Thompson JT, Faler BJ et al (2002) Late fascial closure in lieu of ventral hernia: the next step in open abdomen management. J Trauma 53:843–849
Navsaria PH, Bunting M, Omoshoro-Jones J et al (2003) Temporary closure of open abdominal wounds by the modified sandwich-vacuum pack technique. Br J Surg 90:718–722
Suliburk JW, Ware DN, Balogh Z et al (2003) Vacuum-assisted wound closure achieves early fascial closure of open abdomens after severe trauma. J Trauma 55:1155–1160
Miller PR, Meredith JW, Johnson JC et al (2004) Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg 239:608–614
Stone PA, Hass SM, Flaherty SK et al (2004) Vacuum-assisted fascial closure for patients with abdominal trauma. J Trauma 57:1082–1086
Miller RS, Morris JA Jr, Diaz JJ Jr et al (2005) Complications after 344 damage-control open celiotomies. J Trauma 59:1365–1371
Oetting P, Rau B, Schlag PM (2006) Abdominal vacuum device with open abdomen. Chirurg 77(586):588–593
Cothren CC, Moore EE, Johnson JL et al (2006) One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg 192:238–242
Perez D, Wildi S, Demartines N et al (2007) Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg 205:586–592
Teixeira PG, Salim A, Inaba K et al (2008) A prospective look at the current state of open abdomens. Am Surg 74:891–897
Wondberg D, Larusson HJ, Metzger U et al (2008) Treatment of the open abdomen with the commercially available vacuum-assisted closure system in patients with abdominal sepsis: low primary closure rate. World J Surg 32:2724–2729. doi:10.1007/s00268-008-9762-y
Ozguc H, Paksoy E, Ozturk E (2008) Temporary abdominal closure with the vacuum pack technique: a 5-year experience. Acta Chir Belg 108:414–419
Kritayakirana K, Maggio PM, Brundage S et al (2010) Outcomes and complications of open abdomen technique for managing non-trauma patients. J Emerg Trauma Shock 3:118–122
Acosta S, Bjarnason T, Petersson U et al (2011) Multicentre prospective study of fascial closure rate after open abdomen with vacuum and mesh-mediated fascial traction. Br J Surg 98:735–743
MacLean AA, O’Keeffe T, Augenstein J (2008) Management strategies for the open abdomen: survey of the American Association for the Surgery of Trauma membership. Acta Chir Belg 108:212–218
Rutherford EJ, Skeete DA, Brasel KJ (2004) Management of the patient with an open abdomen: techniques in temporary and definitive closure. Curr Probl Surg 41:811–876
Mattox KL (1997) Introduction, background, and future projections of damage control surgery. Surg Clin North Am 77:753–759
Ghimenton F, Thomson SR, Muckart DJ et al (2000) Abdominal content containment: practicalities and outcome. Br J Surg 87:106–109
Nagy KK, Fildes JJ, Mahr C et al (1996) Experience with three prosthetic materials in temporary abdominal wall closure. Am Surg 62:331–335
Boele van Hensbroek P, Wind J, Dijkgraaf MG et al (2009) Temporary closure of the open abdomen: a systematic review on delayed primary fascial closure in patients with an open abdomen. World J Surg 33:199–207. doi:10.1007/s00268-008-9867-3
Brock WB, Barker DE, Burns RP (1995) Temporary closure of open abdominal wounds: the vacuum pack. Am Surg 61:30–35
Sammons A, Delgado A, Cheatham ML (2009) In vitro pressure manifold distribution evaluation of ABThera active abdominal therapy, V.A.C. abdominal dressing system, and the Barker’s vacuum packing technique, conducted under dynamic conditions, pp 1–4. http://openabdomen.com, 2010
Vogel TR, Diaz JJ, Miller RS et al (2006) The open abdomen in trauma: do infectious complications affect primary abdominal closure? Surg Infect (Larchmt) 7:433–441
Cotton BA, Guy JS, Morris JA Jr et al (2006) The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock 26:115–121
Cotton BA, Gunter OL, Isbell J et al (2008) Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma 64:1177–1182
Holcomb JB, Jenkins D, Rhee P et al (2007) Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 62:307–310
Fouche Y, Sikorski R, Dutton RP (2010) Changing paradigms in surgical resuscitation. Crit Care Med 38:S411–S420
Cotton BA, Au BK, Nunez TC et al (2009) Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 66:41–48
De Laet I, Hoste E, Verholen E et al (2007) The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med 33:1811–1814
Abouassaly CT, Dutton WD, Zaydfudim V et al (2010) Postoperative neuromuscular blocker use is associated with higher primary fascial closure rates after damage control laparotomy. J Trauma 69:557–561
Murphy GS, Vender JS (2001) Neuromuscular-blocking drugs: use and misuse in the intensive care unit. Crit Care Clin 17:925–942
Moore EE, Jones TN (1986) Benefits of immediate jejunostomy feeding after major abdominal trauma: a prospective, randomized study. J Trauma 26:874–881
Moore FA, Feliciano DV, Andrassy RJ et al (1992) Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg 216:172–183
Kudsk KA, Croce MA, Fabian TC et al (1992) Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 215:503–511
Cheatham ML, Safcsak K, Brzezinski SJ et al (2007) Nitrogen balance, protein loss, and the open abdomen. Crit Care Med 35:127–131
Cothren CC, Moore EE, Ciesla DJ et al (2004) Postinjury abdominal compartment syndrome does not preclude early enteral feeding after definitive closure. Am J Surg 188:653–658
Collier B, Guillamondegui O, Cotton B et al (2007) Feeding the open abdomen. JPEN J Parenter Enteral Nutr 31:410–415
Dissanaike S, Pham T, Shalhub S et al (2008) Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg 207:690–697
Byrnes MC, Reicks P, Irwin E (2010) Early enteral nutrition can be successfully implemented in trauma patients with an open abdomen. Am J Surg 199:359–362
Smith BP, Adams RC, Doraiswamy VA et al (2010) Review of abdominal damage control and open abdomens: focus on gastrointestinal complications. J Gastrointest Liver Dis 19:425–435
Schrag SP, Sharma R, Jaik NP et al (2007) Complications related to percutaneous endoscopic gastrostomy (PEG) tubes: a comprehensive clinical review. J Gastrointest Liver Dis 16:407–418
Ericsson CD, Fischer RP, Rowlands BJ et al (1989) Prophylactic antibiotics in trauma: the hazards of underdosing. J Trauma 29:1356–1361
Reed RL II, Ericsson CD, Wu A et al (1992) The pharmacokinetics of prophylactic antibiotics in trauma. J Trauma 32:21–27
Fabian TC (2002) Infection in penetrating abdominal trauma: risk factors and preventive antibiotics. Am Surg 68:29–35
Toschlog EA, Blount KP, Rotondo MF et al (2003) Clinical predictors of subtherapeutic aminoglycoside levels in trauma patients undergoing once-daily dosing. J Trauma 55:255–260
Hakobyan RV, Mkhoyan GG (2008) Epidural analgesia decreases intraabdominal pressure in postoperative patients with primary intra-abdominal hypertension. Acta Clin Belg 63:86–92
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Regner, J.L., Kobayashi, L. & Coimbra, R. Surgical Strategies for Management of the Open Abdomen. World J Surg 36, 497–510 (2012). https://doi.org/10.1007/s00268-011-1203-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1203-7