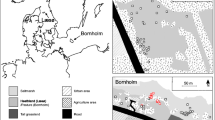
Abstract
Ant colonies may have a single or several reproductive queens (monogyny and polygyny, respectively). In polygynous colonies, colony reproduction may occur by budding, forming multinest, polydomous colonies. In most cases, budding leads to strong genetic structuring within populations, and positive relatedness among nestmates. However, in a few cases, polydomous populations may be unicolonial, with no structuring and intra-nest relatedness approaching zero. We investigated the spatial organisation and genetic structure of a polygynous, polydomous population of Formica truncorum in Finland. F. truncorum shifts nest sites between hibernation and the reproductive season, which raises the following question: are colonies maintained as genetic entities throughout the seasons, or is the population unicolonial throughout the season? Using nest-specific marking and five microsatellite loci, we found a high degree of mixing between individuals of the population, and no evidence for a biologically significant genetic structuring. The nestmate relatedness was also indistinguishable from zero. Taken together, the results show that the population is unicolonial. In addition, we found that the population has undergone a recent bottleneck, suggesting that the entire population may have been founded by a very limited number of females. The precise causes for unicoloniality in this species remain open, but we discuss the potential influence of intra-specific competition, disintegration of recognition cues and the particular hibernation habits of this species.


Similar content being viewed by others
References
Astruc C, Malosse C, Errard C (2001) Lack of intraspecific aggression in the ant Tetramorium bicarinatum: a chemical hypothesis. J Chem Ecol 27:1229–1248
Banschbach VS, Herbers JM (1996a) Complex colony structure in social insects. I. Ecological determinants and genetic consequences. Evolution 50:285–297
Banschbach VS, Herbers JM (1996b) Complex colony structure in social insects. II. Reproduction, queen-worker conflict, and levels of selection. Evolution 50:298–307
Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F (2001) GENETIX 4.02, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome Populations et Intéractions, Université Montpellier II, France
Beye M, Neumann P, Moritz RFA (1997) Nestmate recognition and the genetic gestalt in the mound-building ant Formica polyctena. Insectes Soc 44:49–58
Beye M, Neumann P, Chapuisat M, Pamilo P, Moritz RFA (1998) Nestmate recognition and the genetic relatedness of nests in the ant Formica pratensis. Behav Ecol Sociobiol 43:67–72
Bolton B (1980) The ant tribe Tetramoriini. The genus Tetramorium Mayr in the oriental and Indo-Australian regions, and in Australia. Bull Br Mus Nat Hist 40:192–384
Boomsma JJ, Brouwer AH, Van Loon AJ (1990) A new polygynou Lasius species (Hymenoptera: Formicidae) from Central Europe. II. Allozymatic confirmation of specific status and social structure. Insectes Soc 37:363–375
Boomsma JJ, Nielsen J, Sundström L, Oldham NJ, Tentschert J, Petersen HC, Morgan ED (2003) Informational constraint on optimal sex allocation in ants. Proc Natl Acad Sci USA 100:8799–8804
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton
Bourke AFG, Heinze J (1994) The ecology of communal breeding: the case of multiple-queen leptothoracine ants. Philos Trans R Soc Lond Ser B 345:359–372
Breed MD, Garry MF, Pearce AN, Hibbard BE, Bjostad LB, Page RE Jr (1995) The role of wax comb in honey bee nestmate recognition. Anim Behav 50:489–496
Bulmer MG (1983) Sex ratio theory in social insects with swarming. J Theor Biol 100:329–339
Chapman RE, Bourke AFG (2001) The influence of sociality on the conservation biology of social insects. Ecol Lett 4:650–662
Chapuisat M (1996) Characterization of microsatellite loci in Formica lugubris B and their variability in other ant species. Mol Ecol 5:599–601
Chapuisat M, Goudet J, Keller L (1997) Microsatellite reveal high population viscosity and limited dispersal in the ant Formica paralugubris. Evolution 51:475–482
Clément JL, Bonavita-Courgourdan AB, Lange C (1987) Nestmate recognition and cuticular hydrocarbons in Camponotus vagus Scop.In: Eder J, Rembold H (eds) Chemistry and biology of social insects. Perperny, Munich, pp 473–474
Collingwood CA (1979) The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Scandinavian Science, Klampenborg
Cornuet JM, Luikart G (1996) Description and power analysis of two test for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Crozier RH (1979) Genetics of sociality. In: Hermann HR (ed) Social insects, vol I. Academic, New York, pp 223–286
Crozier RH, Pamilo P (1996) Evolution of social insects colonies: sex allocation and kin selection. Oxford University Press, Oxford
Debout G, Provost E, Renucci M, Tirard A, Schatz B, McKey D (2003) Colony structure in a plant-ant: behavioural, chemical and genetic study of polydomy in Cataulacus mckeyi (Myrmicinae). Oecologia 137:195–204
DeHeer CJ, Backus VL, Herbers JM (2001) Sociogenetic responses to ecological variation in the ant Myrmica punctiventris are context dependent. Behav Ecol Sociobiol 49:375–386
Espadaler X, Rey S (2001) Biological constraints and colony founding in the polygynous invasive ant Lasius neglectus (Hymenoptera, Formicidae). Insectes Soc 48:159–164
Giraud T, Pedersen JS, Keller L (2002) Evolution of supercolonies: the Argentine ants of southern Europe. Proc Natl Acad Sci USA 99:6075–6079
Goudet J, Raymond M, De Meeüs T, Rousset F (1996) Testing differentiation in diploid populations. Genetics 144:1933–1940
Gyllenstrand N (2002) Effects of social organization on spatial genetic structures in Formica ants. PhD Thesis, Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology
Gyllenstrand N, Gertsch PJ, Pamilo P (2002) Polymorphic microsatellite DNA markers in the ant Formica exsecta. Mol Ecol Notes 2:67–69
Gyllenstrand P, Seppä P (2003) Conservation genetics of the wood ant, Formica lugubris, in a fragmented landscape. Mol Ecol 12:2931–2940
Hamilton WD (1964) The genetical evolution of social behaviour. J Theor Biol 7:1–52
Helanterä H (2004) Kinship and conflicts over male production in Formica ants. PhD Thesis, University of Helsinki (in press)
Herbers JM (1986) Nest site limitation and facultative polygyny in the ant Leptothorax longispinosus. Behav Ecol Sociobiol 19:115–122
Higashi S (1976) Nest proliferation by budding and nest growth pattern in Formica (Formica) yessensis in Ishikari Shore. J Fac Sci Hokkaido Univ Ser VI Zool 20:359–389
Higashi S (1978a) Analysis of of internest drifting in a super colonial ant Formica (Formica) yessensis by individually marked workers. Kontyû 46:176–191
Higashi S (1978b) Task and areal conservatism and internest drifting in a red wood ant Formica (Formica) yessensis Forel. Jpn J Ecol 28:257–264
Hölldobler B, Lumsden CJ (1980) Territorial strategies in ants. Science 210:732–739
Hölldobler B, Wilson EO (1977) The number of queens: an important trait in evolution. Naturwissenschaften 64:8–15
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge, Mass
Holway DA (1999) Competitive mechanism underlying the displacement of native ants by the invasive Argentine ant. Ecology 80:238–251
Holway DA, Case JT (2001) Effect of colony-level variation on competitive ability in the invasive Argentine ant. Anim Behav 61:1181–1192
Holway DA, Suarez AV, Case JT (1998) Loss of intraspecific aggression in the success of a widespread invasive social insect. Science 282:949–952
Human KG, Gordon DM (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105:405–412
Ito M (1973) Seasonal population trends and nest structure in a polydomous ant, Formica (Formica) yessensis Forel. J Fac Sci Hokkaido Univ Ser VI Zool 19:270–293
Ito M, Imamura S (1974) Observations on the nuptial flight and internidal relationship in a polydomous ant, Formica (Formica) yessensis Forel. J Fac Sci Hokkaido Univ Ser VI Zool 19:681–694
Kaufmann B, Boomsma JJ, Passera L, Petersen KN (1992) Relatedness and inbreeding in a French population of the unicolonial ant Iridomyrmex humilis (Mayr). Insectes Soc 39:195–213
Keller L (1988) Evolutionary implications of polygyny in the Argentine ant, Iridomyrmex humilis (Mayr) (Hymenoptera: Formicidae): an experimental study. Anim Behav 36:159–165
Keller L (1993a) The assessment of reproductive success of queens in ants and social insects. Oikos 67:177–180
Keller L (1993b) Queen number and sociality in insects. Oxford University Press, Oxford
Keller L (1995) Social life: the paradox of multiple-queen colonies. Trends Ecol Evol 10:353–360
Keller L, Ross KG (1998) Selfish genes: a green beard in the red fire ant. Nature 394:573–575
Liautard C, Keller L (2001) Restricted effective queen dispersal at a microgeographic scale in polygynous populations of the ant Formica exsecta. Evolution 55:2484–2492
Macewicz S (1979) Some consequences of Fisher’s sex ratio principle for social Hymenoptera that reproduce by colony fission. Am Nat 113:363–371
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Markin GP (1970) The seasonal life cycle of the Argentine ant, Iridomyrmex humilis, in southern California. Ann Entomol Soc Am 63:1238–1242
Maruyama T, Fuerst PA (1985) Population bottlenecks and nonequilibrium models in population genetics. II. Number of alleles in a small population that was formed by a recent bottleneck. Genetics 111:675–689
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Nielsen J, Boomsma JJ, Oldham NJ, Petersen HC, Morgan ED (1999) Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insectes Soc 46:58–65
Nonacs P (1988) Queen number in colonies of social Hymenoptera as a kin-selected adaptation. Evolution 42:566–580
Nonacs P (1993) The effects of polygyny and colony life history on optimal sex investissment. In Keller L (ed) Queen number and sociality in insects. Oxford University Press, Oxford, pp 110–131
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Pamilo P (1982) Genetic population structure in polygynous Formica ants. Heredity 48:95–106
Pamilo P (1983) Genetic differentiation within subdivided population of Formica ants. Evolution 37:1010–1022
Pamilo P (1991) Evolution of colony characteristics in social insects. II. Number of reproductive individuals. Am Nat 138:412–433
Pamilo P, Sundström L, Fortelius W, Rosengren R (1994) Diploid males and colony-level selection in Formica ants. Ethol Ecol Evol 6:221–235
Passera L (1994) Characteristics of tramp species. In: Williams DF (ed) Exotic ants: biology, impact, and control of introduced species. Westview, Boulder, Colo, pp 23–43
Passera L, Gilbert M, Aron S (2001) Social parasitism in ants: effect of the inquiline parasite Plagiolepis xene St. on queen distribution and worker production of its host plagiolepis pygmaea Latr. Insectes Soc 48:1–82
Pedersen JS, Boomsma JJ (1999) Genetic analysis of colony structure in polydmous and polygynous ant populations. Biol J Linn Soc 66:115–144
Pirk CWW, Neumann P, Moritz RFA, Pamilo P (2001) Intranest relatedness and nestmate recognition in the meadow ant Formica pratensis (R.). Behav Ecol Sociobiol 49:366–374
Piry S, Luikart G, Cornuet JM (1999) Bottleneck: a computer program for detecting recent reductions in effective population size from allele frequency data. J Hered 90:502–503
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic markers. Evolution 242:258–275
Queller DC, Strassman JE (1998) Kin selection and social insects. Bioscience 48:165–175
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rosengren R (1986) Competition and coexistence in an insular ant community—a manipulation experiment (Hymenoptera: Formicidae). Ann Zool Fenn 23:297–302
Rosengren R, Pamilo P (1983) The evolution and polygyny and polydomy in mound building Formica ants. Acta Entomol Fenn 42:65–77
Rosengren R, Cherix D, Pamilo P (1985) Insular ecology of the red wood ant Formica truncorum Fabr. I. Polydomous nesting, population size and foraging. Mitt Schweiz Entomol Ges 58:147–175
Rosengren R, Cherix D, Pamilo P (1986) Insular ecology of the red wood ant Formica truncorum Fabr. I. Distribution, reproductive strategy and competition. Mitt Schweiz Entomol Ges 59:63–94
Ross KG (1993) The breeding system of the fire ant Solenopsis invicta: effects of colony genetic structure. Am Nat 141:554–576
Ross KG, Keller L (1998) Genetic control of social organization in an ant. Proc Natl Acad Sci USA 95:14232–14237
Ross KG, Vargo EL, Keller L (1996) Social evolution in a new environment: the case of introduced fire ants. Proc Natl Acad Sci USA 93:3021–3025
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Seppä P, Pamilo P (1995) Gene flow and population viscosity in Myrmica ants. Heredity 74:200–209
Seppä P, Walin L (1996) Sociogenetic organization of the red ant Myrmica rubra. Behav Ecol Sociobiol 38:207–217
Seppä P, Sundström L, Punttila P (1995) Facultative polygyny and habitat succession in boreal ants. Biol J Linn Soc 56:533–551
Snyder LE, Herbers JM (1991) Polydomy and sexual allocation ratios in the ant Myrmica punctiventris. Behav Ecol Sociobiol 28:409–415
Sundström L (1989) Genetic relatedness and population structure in Formica truncorum Fabr. (Hymenoptera: Formicidae). Actes Coll Insectes Soc 5:93–100
Sundström L (1993) Genetic population structure and sociogenetic organization in Formica truncorum. Behav Ecol Sociobiol 33:345–354
Tsutsui ND, Case TJ (2001) Population genetics and colony structure of the Argentine ant (Linepithema humile) in its native and introduced ranges. Evolution 55:976–985
Tsutsui ND, Suarez AV, Holway DA, Case TJ (2000) Reduced genetic variation and the success of invasive species. Proc Natl Acad Sci USA 97:5948–5953
Tsutsui ND, Suarez AV, Grosberg-RK (2003) Genetic diversity, asymmetrical aggression, and recognition in a widespread invasive species. Proc Natl Acad Sci USA 100:1078–1083
Van der Hammen T, Pedersen JS, Boomsma JJ (2002) Convergent development of low-relatedness supercolonies in Myrmica ants. Heredity 89:83–89
Weir BS (1979) Inferences about linkage disequilibrium. Biometrics 35:235–254
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wenseleers T, Helanterä H, Hart AG, Ratnieks FLW (2004) Worker reproduction and policing in insect societies. An ESS analysis. J Evol Biol 17:1035–1047
Acknowledgements
We are grateful to Katja Bargum, Christine Johnson, Soile Kupiainen, Kristian Lindqvist, Petro Pynnönen, Elina Ranta, Ayman Abu Saleh and Jaime de Vizcaya for their help in the field and in the laboratory, and to Cathy Liautard, Marie-Hélène Muller, Jes Pedersen, and especially Pekka Pamilo, for fruitful discussions and comments on early versions of this paper. We also thank Niclas Gyllenstrand, Pekka Pamilo and Perttu Seppä for allowing us to perform a bottleneck analysis on their data set, Marita Rosengren for “logistic” support and three anonymous referees, whose comments greatly improved the quality of this paper. This work was funded by the Center for International Mobility of Finland (CIMO), and by the Academy of Finland (grants 42725, 173227 and 206505). Marianne Elias acknowledges the financial support provided through the European Community’s Improving Human Potential Programme under contract HPRN-CT-2000-00052, INSECTS network, and under contract HPMF-CT-2002-01781 (Marie Curie individual fellowship). The experiments carried out in this study comply with the current laws of Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rainer Rosengren is deceased
Communicated by R.F.A. Moritz
Rights and permissions
About this article
Cite this article
Elias, M., Rosengren, R. & Sundström, L. Seasonal polydomy and unicoloniality in a polygynous population of the red wood ant Formica truncorum. Behav Ecol Sociobiol 57, 339–349 (2005). https://doi.org/10.1007/s00265-004-0864-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0864-8