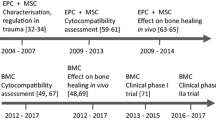
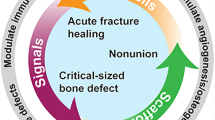
Abstract
Segmental bone defect management is among the most demanding issues in orthopaedics and there is a great medical need for establishing an appropriate treatment option. Tissue transfer, including bone autografts or free flaps, depending on the size of the bone deficiency, is currently the “gold standard” for treatment of such defects. Osteogenic cells in combination with adequate growth factors and a suitable scaffold, from the aspect of osteoinductivity, osteoconductivity and mechanical stability, are mandatory to successfully restore a bone defect as determined in the “diamond concept”. Our current knowledge on this topic is limited and mostly based on retrospective studies, case reports and a few small randomised clinical trials due to the lack of large and accurately designed randomised clinical trials using novel approaches to regenerative orthopaedics. However, preclinical research on different animal models for critical size defects is abundant, showing emerging candidate cells and cytokines for defect rebridgement. In this article we provide an overview on existing clinical studies and promising preclinical experiments that utilised osteogenic cells, growth factors and biomaterials, as well as their combination for repair of segmental bone defects.

Similar content being viewed by others
References
Keating JF, Simpson AH, Robinson CM (2005) The management of fractures with bone loss. J Bone Joint Surg (Br) 87:142–150
Molina CS, Stinner DJ, Obremsky WT (2014) Treatment of traumatic segmental long-bone defects. J Bone Joint Surg Am 2(4):e1
Cappendijk VC, van de Ven KP, Madern GC, Haverlag R, van Vugt AB, Hazebroek FW (2000) Strength of youth: conservative treatment of segmental bone defect in children. J Trauma 49:1123–1125
Hinsche AF, Giannoudis PV, Matthews SJE, Smith RM (2003) Spontaneous healing of a 14 cm diaphyseal cortical defect of the tibia. Injury 34:385–388
Bumbasirevic M, Stevanovic M, Bumbasirevic V, Lesic A, Atkinson HD (2014) Free vascularised fibular grafts in orthopaedics. Int Orthop 38:1277–1282
Ilizarov GA (1992) The replacement of long tubular bone defects by lengthening distraction osteotomy of one of the fragments. 1969. Clin Orthop Relat Res 280:7–10
Gubin AV, Borzunov DY, Malkova TA (2013) The Ilizarov paradigm: thirty years with the Ilizarov method, current concerns and future research. Int Orthop 37:1533–1539
Paley D, Maar DC (2000) Ilizarov bone transport treatment for tibial defects. J Orthop Trauma 14:76–85
Burg KJ, Porter S, Kellam JF (2000) Biomaterial developments for bone tissue engineering. Biomaterials 21:2347–59
Ronca A, Guarino V, Raucci MG, Salamanna F, Martini L, Zeppetelli S, Fini M, Kon E, Filardo G, Marcacci M, Ambrosio L (2014) Large defect-tailored composite scaffolds for in vivo bone regeneration. J Biomater Appl 29:715–727
Tiedeman JJ, Garvin KL, Kile TA, Connolly JF (1995) The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics 18:1153–1158
Gao TJ, Lindholm TS, Kommonen B, Ragni P, Paronzini A, Lindholm TC, Jalovaara P, Urist MR (1997) The use of a coral composite implant containing bone morphogenetic protein to repair a segmental tibial defect in sheep. Int Orthop 21:194–200
Leijten J, Chai YC, Papantoniou I, Geris L, Schrooten J, Luyten FP (2014) Cell based advanced therapeutic medicinal products for bone repair: keep it simple? Adv Drug Deliv Rev. doi:10.1016/j.addr.2014.10.025
Moreira Teixeira LS, Patterson J, Luyten FP (2014) Skeletal tissue regeneration: where can hydrogels play a role? Int Orthop 38:1861–1876
Chai YC, Kerckhofs G, Roberts SJ, Van Bael S, Schepers E, Vleugels J, Luyten FP, Schrooten J (2012) Ectopic bone formation by 3D porous calcium phosphate-Ti6Al4V hybrids produced by perfusion electrodeposition. Biomaterials 33:4044–4058
Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R (2007) Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 13:947–955
Masquelet AC, Begue T (2010) The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am 41:27–37
Aurégan JC, Bégué T (2014) Induced membrane for treatment of critical sized bone defect: a review of experimental and clinical experiences. Int Orthop 38:1971–1978
Hinsenkamp M, Collard JF (2015) Growth factors in orthopaedic surgery: demineralized bone matrix versus recombinant bone morphogenetic proteins. Int Orthop 39:137–147
Fassbender M, Minkwitz S, Thiele M, Wildemann B (2014) Efficacy of two different demineralised bone matrix grafts to promote bone healing in a critical-size-defect: a radiological, histological and histomorphometric study in rat femurs. Int Orthop 38:1963–1969
Donegan DJ, Scolaro J, Matuszewski PE, Mehta S (2011) Staged bone grafting following placement of an antibiotic spacer block for the management of segmental long bone defects. Orthopedics 34:e730–e735
Giannoudis PV, Einhorn TA, Marsh D (2007) Fracture healing: the diamond concept. Injury 38(Suppl 4):S3–S6
Zhu W, Xiao J, Wang D, Liu J, Xiong J, Liu L, Zhang X, Zeng Y (2009) Experimental study of nano-HA artificial bone with different pore sizes for repairing the radial defect. Int Orthop 33:567–571
Deng M, Zhang B, Wang K, Liu F, Xiao H, Zhao J, Liu P, Li Y, Lin F, Wang Y (2011) Mechano growth factor E peptide promotes osteoblasts proliferation and bone-defect healing in rabbits. Int Orthop 35:1099–1106
Tölli H, Kujala S, Jämsä T, Jalovaara P (2011) Reindeer bone extract can heal the critical-size rat femur defect. Int Orthop 35:615–622
Vukicevic S, Oppermann H, Verbanac D, Jankolija M, Popek I, Curak J, Brkljacic J, Pauk M, Erjavec I, Francetic I, Dumic-Cule I, Jelic M, Durdevic D, Vlahovic T, Novak R, Kufner V, Bordukalo Niksic T, Kozlovic M, Banic Tomisic Z, Bubic-Spoljar J, Bastalic I, Vikic-Topic S, Peric M, Pecina M, Grgurevic L (2014) The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int Orthop 38:635–647
Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, Grgurevic L, Trkulja V, Bagi CM, Vukicevic S (2015) The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 70:73–86
Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW (2009) The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30:2149–2163
Gazdag AR, Lane JM, Glaser D, Forster RA (1995) Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg 3:1–8
Hernigou P, Desroches A, Queinnec S, Flouzat Lachaniette CH, Poignard A, Allain J, Chevallier N, Rouard H (2014) Morbidity of graft harvesting versus bone marrow aspiration in cell regenerative therapy. Int Orthop 38:1855–1860
Hernigou P (2015) Bone transplantation and tissue engineering, part III: allografts, bone grafting and bone banking in the twentieth century. Int Orthop. doi:10.1007/s00264-015-2669-y
Sassard WR, Eidman DK, Gray PM, Block JE, Russo R, Russell JL, Taboada EM (2000) Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: avoiding second site autologous bone harvest. Orthopedics 23:1059–1064
Stafford PR, Norris BL (2010) Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury 41(Suppl 2):S72–S77
Porter RM, Liu F, Pilapil C, Betz OB, Vrahas MS, Harris MB, Evans CH (2009) Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res 27:42–49
Miller MA, Ivkovic A, Porter R, Harris MB, Estok DM 2nd, Smith RM, Evans CH, Vrahas MS (2011) Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop 35:599–605
Martinovic S, Borovecki F, Miljavac V, Kisic V, Maticic D, Francetic I, Vukicevic S (2006) Requirement of a bone morphogenetic protein for the maintenance and stimulation of osteoblast differentiation. Arch Histol Cytol 69:23–36
Dumic-Cule I, Brkljacic J, Rogic D, Bordukalo Niksic T, Tikvica Luetic A, Draca N, Kufner V, Trkulja V, Grgurevic L, Vukicevic S (2014) Systemically available bone morphogenetic protein two and seven affect bone metabolism. Int Orthop 38:1979–1985
Patterson TE, Kumagai K, Griffith L, Muschler GF (2008) Cellular strategies for enhancement of fracture repair. J Bone Joint Surg Am 90(Suppl 1):111–119
Stangenberg L, Schaefer DJ, Buettner O, Ohnolz J, Möbest D, Horch RE, Stark GB, Kneser U (2005) Differentiation of osteoblasts in three-dimensional culture in processed cancellous bone matrix: quantitative analysis of gene expression based on real-time reverse transcription-polymerase chain reaction. Tissue Eng 11:855–864
Bruder SP, Fink DJ, Caplan AI (1994) Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem 56:283–294
Yamanaka S (2012) Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10:678–684
Kitoh H, Kawasumi M, Kaneko H, Ishiguro N (2009) Differential effects of culture-expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthenings. J Pediatr Orthop 29:643–649
Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N (2007) Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone 40:522–528
Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87:1430–1437
Léotot J, Lebouvier A, Hernigou P, Bierling P, Rouard H, Chevallier N (2014) Bone-forming capacity and biodistribution of bone marrow-derived stromal cells directly loaded into scaffolds: a novel and easy approach for clinical application of bone regeneration. Cell Transplant. doi:10.3727/096368914X685276
Hernigou P, Pariat J, Queinnec S, Homma Y, Flouzat Lachaniette CH, Chevallier N, Rouard H (2014) Supercharging irradiated allografts with mesenchymal stem cells improves acetabular bone grafting in revision arthroplasty. Int Orthop 38:1913–1921
Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, Jacobson MS, Heeschen C (2008) Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant 16:1059–1069
Hendrich C, Franz E, Waertel G, Krebs R, Jäger M (2009) Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev (Pavia) 1:e32
Gessmann J, Köller M, Godry H, Schildhauer TA, Seybold D (2012) Regenerate augmentation with bone marrow concentrate after traumatic bone loss. Orthop Rev (Pavia) 4:e14
Petri M, Namazian A, Wilke F, Ettinger M, Stübig T, Brand S, Bengel F, Krettek C, Berding G, Jagodzinski M (2013) Repair of segmental long-bone defects by stem cell concentrate augmented scaffolds: a clinical and positron emission tomography–computed tomography analysis. Int Orthop 37:2231–2237
Jäger M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, Hendrich C, Krauspe R (2011) Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res 29:173–180
The BMP2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group, Govender S, Csimma C, Genant HK, Valentin-Orpan A (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of 450 patients. J Bone Joint Surg-Am 84-A:2123–2134
Pecina M, Vukicevic S (2007) Biological aspects of bone, cartilage and tendon regeneration. Int Orthop 31:719–720
Pecina M, Haspl M, Jelic M, Vukicevic S (2003) Repair of a resistant tibial non-union with a recombinant bone morphogenetic protein-7 (rhBMP-7). Int Orthop 27:320–321
Bishop GB, Einhorn TA (2007) Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop 31:721–727
Courvoisier A, Sailhan F, Laffenêtre O, Obert L; French Study Group of BMP in Orthopedic Surgery (2014) Bone morphogenetic protein and orthopaedic surgery: can we legitimate its off-label use? Int Orthop 38:2601–2605
Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A (2006) BMP-2 Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study Group, Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am 88:1431–1441
Geesink RG, Hoefnagels NH, Bulstra SK (1999) Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg (Br) 81:710–718
Pecina M, Giltaij LR, Vukicevic S (2001) Orthopaedic applications of osteogenic protein-1 (BMP-7). Int Orthop 25:203–208
Bilic R, Simic P, Jelic M, Stern-Padovan R, Dodig D, Pompe van Meerdervoort H, Martinovic S, Ivankovic D, Pecina M, Vukicevic S (2006) Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. Int Orthop 30:128–134
Anticevic D, Jelic M, Vukicevic S (2006) Treatment of a congenital pseudarthrosis of the tibia by osteogenic protein-1 (bone morphogenetic protein-7): a case report. J Pediatr Orthop B 15:220–221
Das SP, Ganesh S, Pradhan S, Singh D, Mohanty RN (2014) Effectiveness of recombinant human bone morphogenetic protein-7 in the management of congenital pseudoarthrosis of the tibia: a randomised controlled trial. Int Orthop 38:1987–1992
Soballe K, Hansen ES, B-Rasmussen H, Pedersen CM, Bunger C (1992) Bone graft incorporation around titanium alloy and hydroxyapatite coated implants in dogs. Clin Orthop 274:282–293
Vukicevic S, Grgurevic L (2009) BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev 20:441–448
Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S (2006) Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem 281:25509–25521
Song K, Krause C, Shi S, Patterson M, Suto R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, Ten Dijke P, Alaoui-Ismaili MH (2010) Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem 285:12169–12180
Allendorph GP, Isaacs MJ, Kawakami Y, Belmonte JC, Choe S (2007) BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry 46:12238–12247
Sieber C, Schwaerzer GK, Knaus P (2008) Bone morphogenetic protein signaling is fine-tuned on multiple levels. In: Vukicevic S, Sampath TK (eds) Bone morphogenetic proteins: from local to systemic therapeutics. Birkauser, Basel, pp 81–114
Korchynskyi O, van Bezooijen RL, Lowik CWGM, ten Dijke P (2004) Bone morpho- genetic protein receptors and their nuclear effectors in bone formation. In: Vukicevic S, Sampath TK (eds) Bone morphogenetic proteins: regeneration of bone and beyond. Birkauser, Basel, pp 9–114
Saremba S, Nickel J, Seher A, Kotzsch A, Sebald W, Mueller TD (2008) Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J 275:172–183
Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M (2013) Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spinal fusion: a systematic review and meta-analysis. Ann Intern Med 158:890–902
Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, Stewart LA (2013) Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med 158:877–889
Minear S, Leucht P, Miller S, Helms JA (2010) rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res 25:1196–1207
Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, Yabe Y, Kumegawa M, Hakeda Y (2000) Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 27:479–486
Urist MR (1965) Bone: formation by autoinduction. Science 150:893–899
Little DG, McDonald M, Bransford R, Godfrey CB, Amanat N (2005) Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J Bone Miner Res 20:2044–2052
Pećina M, Vukičević S (2014) Tissue engineering and regenerative orthopaedics (TERO). Int Orthop 38:1757–1760
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme [FP7/2007-2013] under grant agreement no. HEALTH-F4-2011-279239. This work has also been supported in part by the Croatian Science Foundation project 08/5 BONE6-BIS.
Conflict of interest
M. Jankolija and I.P. are employees of Genera Research. S.V. is the founder of Genera Research. I.D.C., M.P., M. Jelic and L.G. declare that they have no conflict of interest and certify that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing, arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dumic-Cule, I., Pecina, M., Jelic, M. et al. Biological aspects of segmental bone defects management. International Orthopaedics (SICOT) 39, 1005–1011 (2015). https://doi.org/10.1007/s00264-015-2728-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2728-4