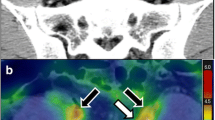
Abstract
Purpose
The objective of the study was to systematically assess aortic inflammation in patients with granulomatosis with polyangiitis (GPA) using 18F-2-deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography (PET)/CT.
Methods
Aortic inflammation was studied in PET/CT scans obtained from 21 patients with GPA; 14 patients with sarcoidosis were included as disease controls, 7 patients with stage I or II head and neck carcinoma ascertained during routine clinical practice were used as healthy controls (HC) and 5 patients with large vessel vasculitis (LVV) were used as positive controls. Aortic 18F-FDG uptake was expressed as the blood-normalized maximum standardized uptake value (SUVmax), known as the target to background ratio (mean TBRmax).
Results
The mean TBRmax (interquartile range) of the aorta in patients with GPA, sarcoidosis, HC and LVV were 1.75 (1.32–2.05), 1.62 (1.54–1.74), 1.29 (1.22–1.52) and 2.03 (1.67–2.45), respectively. The mean TBRmax was significantly higher in patients suffering from GPA or LVV compared to HC (p < 0.05 and p < 0.005, respectively) and tended to be higher in patients suffering from sarcoidosis, but this did not reach statistical significance (p = 0.098). The mean TBRmax of the most diseased segment was significantly higher compared to HC [1.57 (1.39–1.81)] in LVV patients [2.55 (2.22–2.82), p < 0.005], GPA patients [2.17 (1.89–2.83), p < 0.005] and patients suffering from sarcoidosis [2.04 (1.88–2.20), p < 0.05]. In GPA patients, the mean TBRmax of the aorta was significantly higher in patients with previous renal involvement [2.01 (1.69–2.53)] compared to patients without renal involvement in the past [1.60 (1.51–1.80), p < 0.05]. Interrater reproducibility with a second reader was high (all intraclass correlation coefficients >0.9).
Conclusion
Patients suffering from GPA show marked aortic FDG uptake.
Similar content being viewed by others
Granulomatosis with polyangiitis (GPA, Wegener’s) is an inflammatory disease entity affecting small to medium vessels. It is, together with microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA, Churg-Strauss syndrome), characterized by the presence of antineutrophil cytoplasmic antibodies (ANCA) and they are frequently grouped together under the term ANCA-associated vasculitis (AAV) [1]. Sarcoidosis is a multisystemic disease with unknown aetiology [2]. These diseases share a common pathology of granuloma formation, although granulomas are noncaseating in sarcoidosis, while necrotizing in GPA.
Positron emission tomography (PET) scanning with 2-deoxy-2-[18F]fluoro-D-glucose (FDG) is used for detecting high glucose metabolism in malignancies and infectious and autoimmune diseases [3–5]. Recent studies have shown that PET scans show abnormalities in patients with ANCA-associated vasculitis [6, 7]. Coregistration with CT allows the increased FDG uptake to be localized to the underlying anatomy. Aortic FDG uptake had been described incidentally in patients undergoing PET scanning during oncology staging and its potential to measure arterial inflammation has since been investigated [8, 9]. The use of PET scanning has sparked attention in the field of atherosclerosis as the arterial FDG uptake may be an independent predictor of cardiovascular events. Moreover, PET scanning has been evaluated and proven to be an important diagnostic tool in large vessel vasculitis (LVV) [10–13]. Measuring arterial FDG uptake has proven to be a reliable and reproducible method when values are blood-normalized, also known as the target to background ratio (TBR) [14–16].
In our previous study we observed enhanced FDG uptake in the aorta of patients with GPA [17]. Pathologically enhanced FDG uptake may have implications for classification of the patients. At present, the distribution of vessel involvement dictates the differentiation between specifically defined forms of vasculitis [18]. Our previous study did not specifically address the aortic uptake of patients in AAV and aortic uptake was merely assessed in a qualitative manner [17]. The aim of our current study is to systematically assess aortic FDG uptake in patients with AAV compared to positive controls (LVV), disease controls (sarcoidosis) and healthy controls (HC) using 18F-FDG PET/CT.
Materials and methods
Patients
Retrospectively, 21 patients with GPA according to the 2012 revised International Chapel Hill Consensus Conference Nomenclature were included [19]. A PET scan was performed in patients with GPA during a period of suspected disease activity when other tools for evaluation of activity were inconclusive. The possibility of an active bacterial or viral infection was excluded by culture, serology and persistence of symptoms despite empirical antibiotic treatment. Previous renal involvement was determined by a renal biopsy showing pauci-immune necrotizing glomerulonephritis in the past [20]. However, the presence of haematuria in combination with red cell casts, dysmorphic erythrocytes (>10) and/or proteinuria sufficed [21].
Fourteen age- and sex-matched patients with biopsy-proven sarcoidosis according to the consensus statement on sarcoidosis of the American Thoracic Society (ATS)/European Respiratory Society (ERS)/World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) [2] were included as disease controls. The indication for a PET scan in patients with sarcoidosis was the presence of unexplained disease-related disabling symptoms that persisted for at least 1 year [4]. Seven age- and sex-matched patients in whom a PET scan was made during daily clinical practice for oncological staging of stage I or II head and neck carcinoma were included as HC. These patients had not (yet) received systemic chemotherapy or radiation therapy at the time of scanning. Lastly, five patients with giant cell arteritis (GCA) according to the 1990 criteria of the American College of Rheumatology (ACR) were included during a period of active disease as positive controls [22].
All PET scans were performed between December 2006 and March 2014 at the Maastricht University Medical Center. This study was carried out in compliance with the Helsinki Declaration; for this type of study formal consent is not required.
18F-FDG PET/CT
An 18F-FDG PET/CT scan was performed by scanning patients using a PET/CT (Gemini TF™ PET/CT, Philips Healthcare Nederland B.V., The Netherlands) scanner with time-of-flight (TOF) capability, together with a 64-slice Brilliance CT scanner. This scanner has a transverse and axial field of view (FOV) of 57.6 and 18 cm, respectively. The spatial resolution is around 5 mm. Patients were fasting for at least 6 h before the examination. In all patients blood glucose was measured to ensure that the blood glucose was below 10 mmol/l. FDG was injected intravenously and followed by physiological saline (10 ml). The injected total activity of FDG depended on the weight of the patient. The mean injected dose was 200 MBq. After a resting period of 45 min (time needed for uptake of FDG) PET and CT images were acquired from the head to the feet. A low-dose CT scan was performed without intravenous contrast and was used for attenuation correction of the PET images. The PET images were acquired in 5-min bed positions. The complete PET data set was reconstructed iteratively with a reconstruction increment of 5 mm to provide isotropic voxels.
Vascular PET image analysis was performed on a Xeleris 2.0 workstation (Extended Brilliance Workspace™ V4.5.3.40140, Philips Healthcare Nederland B.V., The Netherlands) using previously described, validated and reproducible methods [14, 15, 23, 24]. Briefly, the aorta was divided into ascending, arch, descending and abdominal segments using anatomical landmarks derived from the CT scan. The aorta was demarcated as the region of interest (ROI) on each slice of the CT scan. Maximum body weight-corrected standardized uptake values (SUVmax) were derived from the pixel activity within the ROI on each slice of the PET scan. Slices were screened for nonvascular enhanced uptake in the close vicinity of the aorta that may falsely increase the SUV value, such as the heart or lymph nodes, and slices were censored if present. In addition, the mean blood FDG activity (SUVmean) was measured in the superior vena cava using an ROI of 5 mm on at least 5 slices, and the ROI was placed in the middle of the superior vena cava to ensure that no FDG uptake of the vessel wall was included. Arterial SUVmax values were blood-normalized by dividing the SUVmax of the aorta by the SUVmean of the blood pool in the vena cava, known as the TBRmax.
Aortic FDG uptake was evaluated using (1) the mean TBRmax of the whole aorta, (2) the mean TBRmax of the most diseased segment, defined as the hottest slice and the adjacent slices superior and inferior to it, and (3) the proportion of active slices, defined as a slice with a TBR higher than 1.6 [25–27].
Statistical procedure
Numerical variables were expressed as mean (SD) or as median (interquartile range, IQR) and categorical variables as numbers (percentages). The Kruskal-Wallis test was used to compare quantitative measurements between more than two groups. Dunn’s multiple comparisons posttest analysis was performed to compare individual groups if a statistical difference was found in the Kruskal-Wallis test. To compare quantitative measurements between two unpaired groups, the Mann-Whitney test was performed. Continuous variables were correlated with the Spearman test.
To assess interrater reliability, a second reader performed the measurements and the results were compared using the intraclass correlation coefficient (two-way random and absolute agreement). All statistical analyses were performed using GraphPad Prism version 6.04 for Windows (GraphPad Software, La Jolla, CA, USA) and SPSS statistics for Windows, version 20.0 (IBM, Armonk, NY, USA).
Results
Aortic FDG uptake using FDG PET/CT was assessed in 21 patients with GPA, 14 patients with sarcoidosis, 7 HC and 5 patients with LVV (see Table 1). In total, 89 (10), 97 (16), 97 (8) and 91 (5) slices of the aorta were evaluated in patients with GPA, sarcoidosis, HC and LVV, respectively, of which 2 (SD 4, 2.2 %), 8 (SD 8, 8.2 %), 4 (SD 5, 4.1 %) and 0 (SD 0, 0 %) slices were excluded due to adjacent nonvascular enhanced uptake. The interrater reliability with the second reader was excellent, with all intraclass correlation coefficient values higher than 0.9 (see Table 2).
Differences were observed in the median mean TBRmax of the aorta between patients with LVV [2.03 (1.67–2.45)], patients with GPA [1.75 (1.32–2.05)], patients with sarcoidosis [1.62 (1.54–1.74)] and HC [1.29 (1.22–1.52)]. The mean TBRmax was higher in patients with LVV and GPA compared to HC (p < 0.005 and p < 0.05, respectively). The mean TBRmax tended to be higher in patients with sarcoidosis compared to HC, but this did not reach statistical significance (p = 0.098). This pattern was observed in all anatomical segments of the aorta (see Fig. 1). The median SUVmax values of the aorta were 3.47 (2.37–3.58) in patients with LVV, 1.90 (1.65–2.06) in patients with AAV, 2.03 (1.88–2.51) in patients with sarcoidosis and 1.62 (1.60–2.32) in HC. The median SUVmean values of the superior vena cava were 1.38 (1.30–1.62), 1.09 (0.81–1.30), 1.25 (1.09–1.45) and 1.34 (1.10–2.09] in patients with LVV, AAV, sarcoidosis and HC, respectively.
The median mean TBRmax of the most diseased segment was significantly higher compared to HC [1.57 (1.39–1.81)] in patients with LVV [2.55 (2.22–2.82), p < 0.005], patients with GPA [2.17 (1.89–2.83), p < 0.005] and patients with sarcoidosis [2.04 (1.88–2.20), p < 0.05, see Fig. 2].
The proportion of hot slices was significantly higher compared to HC [3.85 % (0–38.46)] in patients with LVV [98.85 % (72.69–100), p < 0.005] and patients with GPA [69.89 % (31.89–99.50), p < 0.05, see Fig. 3]. The proportion of hot slices was not statistically different in patients with sarcoidosis [49.17 % (34.89–75.58)] compared to HC (p = 0.21).
In patients with GPA, the median mean TBRmax of the aorta was significantly higher in patients with previous renal involvement [2.01 (1.69–2.53)] compared to patients without renal involvement in the past [1.60 (1.51–1.80), p < 0.05, see Fig. 4]. The mean TBRmax of the aorta correlated significantly with the C-reactive protein level at the time of scanning (R = 0.42, p < 0.005).
Discussion
It was demonstrated that PET scans show higher FDG uptake in the aorta in patients with AAV compared to HC. In addition, the most diseased segment was higher in patients with GPA and patients with sarcoidosis compared to HC. Moreover, the aortic uptake in GPA patients with previous renal involvement was higher compared to patients without renal involvement in the past and similar to patients with LVV.
Since the accidental finding of aortic FDG uptake during oncological staging, 18F-FDG PET scanning has been advocated as an imaging tool that reflects the cellular pathology of atherosclerosis rather than the anatomical consequence [28]. The central question remains what underlying process is reflected by the enhanced FDG uptake in the aorta. Currently, the FDG uptake is thought to represent macrophage activity in inflamed intimal atherosclerotic plaques [9, 29]. Several histopathological studies have indeed shown that the intensity of FDG uptake correlates with the number of macrophages observed in the vessel wall [26, 28]. Interestingly, FDG uptake and arterial calcification rarely overlap [30].
The phenomenon of accelerated atherosclerosis is observed in various autoimmune diseases, including GPA [31]. Patients with GPA have a two- to fourfold increased relative risk of coronary artery disease as compared to control subjects, independent of classic risk factors [31]. Long-term mortality in GPA is dominated by cardiovascular events [32]. Recently we have shown that the metabolic syndrome is more prevalent in patients with AAV compared to HC [33]. More specifically, patients with AAV suffer more often from hypertension and an increased waist circumference, while the prevalence of the other criteria for the metabolic syndrome are comparable. In our current study, patients with sarcoidosis more often suffered from overweight and hypertension, while other cardiovascular risk factors were similar.
The finding of enhanced aortic FDG uptake has been studied in parallel in patients with LVV [34]. The spectrum of LVV consists of Takayasu’s arteritis (TA) and GCA. FDG PET scans have been performed in both diseases and have shown high SUV values of the aorta. However, most studies concerning LVV only report the SUV value, which may not be the optimal measure to quantify FDG uptake in the aorta [9, 24, 35]. The TBRmax value has the advantage, to a certain extent, to correct for the FDG uptake in the blood. Recently an effort has been made towards an optimal semi-quantitative approach to measure arterial inflammation in LVV [16]. Interestingly, SUVmax values of the aorta were not increased in patients with GPA compared to the other patient groups (see “Supplementary material”). This has been observed in patients with GCA as well, in which the TBRmax value, but not the SUV value, was significantly higher in patients with GCA compared to HC [16]. Normalization by blood pool activity outperformed the other approaches and permitted the differentiation of patients with GCA from control patients [16].
The distribution of vessel involvement has in the past dictated the differentiation between specifically defined forms of vasculitis [18]. Cases have previously been reported with evident large vessel involvement in patients with small vessel vasculitis, thereby questioning the validity of the differentiation in vessel distribution [36]. Therefore, in the 2012 revised Chapel Hill Consensus Conference, it is noted that there is substantial overlap with respect to arterial involvement [19]. Our results support this notion, as the mean TBRmax values in patients with AAV were comparable to the values measured in patients with GCA.
Does the enhanced aortic FDG uptake in our patients with GPA reflect atherosclerosis or large vessel involvement? The mean TBRmax values measured in our patients with GPA are at the high end of values observed in patients with established cardiovascular disease (see Table 3). Unfortunately, FDG uptake is not specific for processes of either disease. More importantly, should the finding of enhanced aortic FDG uptake prompt a change in treatment? Several studies have shown that the FDG uptake reduces after statin treatment [40, 38]. In addition, Mäki-Petäjä et al. have shown that anti-tumour necrosis factor-α treatment reduces aortic inflammation in patients with rheumatoid arthritis [25]. However, it is currently uncertain whether enhanced FDG uptake in the aorta should be an indication to start treatment with a statin or an immunosuppressive agent. Further research is warranted to assess the clinical significance of aortic inflammation.
Lastly, we have shown that the mean TBRmax value of the most diseased segment of the aorta in patients with sarcoidosis was higher compared to HC, while the mean TBRmax values of the entire aorta were comparable. This suggests that patients with sarcoidosis suffer from aortic inflammation with a focal pattern but not diffuse. Systemic vasculitis in sarcoidosis is uncommon but has been described previously [41].
Our study suffers from several limitations. Due to the retrospective character of the study we were unable to assess other risk factors, such as the lipid profile. However, in a previous study these values were not elevated in our patients with AAV compared to HC [33]. Our patients with stage I or II head and neck carcinoma suffered from less cardiovascular risk factors compared to the patients with GPA and may therefore not be an ideal control group. The mean TBRmax of 1.36 ± 0.16 observed in our HC was slightly lower compared to the values reported in the literature, ranging between 1.49 and 1.60. Recent findings suggest that the pre-scan glucose levels and the circulation times were suboptimal for the evaluation of FDG uptake in the aorta [24].
In conclusion, patients with GPA showed marked FDG uptake in the aorta compared to HC. In addition, the aortic FDG uptake in GPA patients with previous renal involvement was equivalent to the aortic FDG uptake in patients with LVV.
References
Wilde B, van Paassen P, Witzke O, Tervaert JWC. New pathophysiological insights and treatment of ANCA-associated vasculitis. Kidney Int 2011;79:599–612.
Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 1999;14:735–7.
Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJG. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med 2010;51:1937–49.
Mostard RLM, Vöö S, van Kroonenburgh MJPG, Verschakelen JA, Wijnen PAHM, Nelemans PJ, et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med 2011;105:1917–24.
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008;49:480–508.
Ito K, Minamimoto R, Yamashita H, Yoshida S, Morooka M, Okasaki M, et al. Evaluation of Wegener’s granulomatosis using 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Ann Nucl Med 2013;27:209–16.
Soussan M, Abisror N, Abad S, Nunes H, Terrier B, Pop G, et al. FDG-PET/CT in patients with ANCA-associated vasculitis: case-series and literature review. Autoimmun Rev 2014;13:125–31.
Joshi F, Rosenbaum D, Bordes S, Rudd J. Vascular imaging with positron emission tomography. J Intern Med 2011;270:99–109.
Sheikine Y, Akram K. FDG-PET imaging of atherosclerosis: do we know what we see? Atherosclerosis 2010;211:371–80.
Fuchs M, Briel M, Daikeler T, Walker U, Rasch H, Berg S, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging 2012;39:344–53.
Zerizer I, Tan K, Khan S, Barwick T, Marzola M, Rubello D, et al. Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol 2010;73:504–9.
Treglia G, Mattoli M, Leccisotti L, Ferraccioli G, Giordano A. Usefulness of whole-body fluorine-18-fluorodeoxyglucose positron emission tomography in patients with large-vessel vasculitis: a systematic review. Clin Rheumatol 2011;30:1265–75.
Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006;55:131–7.
Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007;50:892–6.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008;49:871–8.
Besson FL, de Boysson H, Parienti JJ, Bouvard G, Bienvenu B, Agostini D. Towards an optimal semiquantitative approach in giant cell arteritis: an (18)F-FDG PET/CT case-control study. Eur J Nucl Med Mol Imaging 2014;41:155–66.
Kemna M, Vandergheynst F, Vöö S, Blocklet D, Nguyen T, Timmermans S, et al. Positron emission tomography scanning in ANCA associated vasculitis. Presse Med 2015;42:742. doi:10.1016/j.lpm.2013.02.216.
Jennette J, Falk R, Andrassy K, Bacon P, Churg J, Gross W, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994;37:187–92.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11.
Hilhorst M, Wilde B, van Breda Vriesman P, van Paassen P, Cohen Tervaert JW, Limburg Renal Registry. Estimating Renal Survival Using the ANCA-Associated GN Classification. J Am Soc Nephrol 2013;24:1371–5.
Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7.
Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–8.
Coulson JM, Rudd JH, Duckers JM, Rees JI, Shale DJ, Bolton CE, et al. Excessive aortic inflammation in chronic obstructive pulmonary disease: an 18F-FDG PET pilot study. J Nucl Med 2010;51:1357–60.
Bucerius J, Mani V, Moncrieff C, Machac J, Fuster V, Farkouh ME, et al. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur J Nucl Med Mol Imaging 2014;41:369–83.
Mäki-Petäjä KM, Elkhawad M, Cheriyan J, Joshi FR, Ostör AJ, Hall FC, et al. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 2012;126:2473–80.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24.
Elkhawad M, Rudd JH, Sarov-Blat L, Cai G, Wells R, Davies LC, et al. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc Imaging 2012;5:911–22.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–11.
Cocker MS, Mc Ardle B, Spence JD, Lum C, Hammond RR, Ongaro DC, et al. Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol 2012;19:1211–25.
Ben-Haim S, Kupzov E, Tamir A, Israel O. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med 2004;45:1816–21.
Cohen Tervaert JW. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol 2013;27:33–44.
Hilhorst M, Wilde B, van Paassen P, Winkens B, van Breda Vriesman P, Cohen Tervaert JW, Limburg Renal Registry. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant 2013;28:373–9.
Petermann Smits DR, Wilde B, Kianersi Adegani M, de Jongh H, van Paassen P, Cohen Tervaert JW. Metabolic syndrome in ANCA-associated vasculitis. Rheumatology (Oxford) 2013;52:197–203.
Blockmans D. PET in vasculitis. Ann N Y Acad Sci 2011;1228:64–70.
Keyes Jr JW. SUV: standard uptake or silly useless value? J Nucl Med 1995;36:1836–9.
Chirinos JA, Tamariz LJ, Lopes G, Del Carpio F, Zhang X, Milikowski C, et al. Large vessel involvement in ANCA-associated vasculitides: report of a case and review of the literature. Clin Rheumatol 2004;23:152–9.
Rudd JH, Myers KS, Bansilal S, Machac J, Woodward M, Fuster V, Farkouh ME, Fayad ZA. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging 2009;2:107–15
Wu YW, Kao HL, Huang CL, Chen MF, Lin LY, Wang YC, et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging 2012;39:399–407.
Noh TS, Moon SH, Cho YS, Hong SP, Lee EJ, Choi JY, et al. Relation of carotid artery 18F-FDG uptake to C-reactive protein and Framingham risk score in a large cohort of asymptomatic adults. J Nucl Med 2013;54:2070–6
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825–31.
Fernandes SR, Singsen BH, Hoffman GS. Sarcoidosis and systemic vasculitis. Semin Arthritis Rheum 2000;30:33–46.
Acknowledgments
We would like to thank Petal Wijnen and Jeroen Mom for their contribution to the acquisition of data.
Ethical approval and informed consent
For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Conflicts of interest
None.
Authors’ contributions
MJK, JB, MD, JWCT and MvK have made substantial contributions to conception and design. MJK, SV, MV and PvP have made substantial contributions to the acquisition of data. MJK has been involved in drafting the manuscript. All authors have made substantial contribution to the analysis and interpretation of data. All authors have been involved in revising the manuscript critically for important intellectual content and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 698 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kemna, M.J., Bucerius, J., Drent, M. et al. Aortic 18F-FDG uptake in patients suffering from granulomatosis with polyangiitis. Eur J Nucl Med Mol Imaging 42, 1423–1429 (2015). https://doi.org/10.1007/s00259-015-3081-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3081-y