Abstract
Purpose
18F-FDG PET is increasingly used for imaging of vessel wall inflammation. However, limited data are available on the impact of methodological variables, i.e. prescan fasting glucose, FDG circulation time and injected FDG dose, and of different FDG uptake parameters, in vascular FDG PET imaging.
Methods
Included in the study were 195 patients who underwent vascular FDG PET/CT of the aorta and the carotids. Arterial standardized uptake values (meanSUVmax), target-to-background ratios (meanTBRmax) and FDG blood-pool activity in the superior vena cava (SVC) and the jugular veins (JV) were quantified. Vascular FDG uptake values classified according to the tertiles of prescan fasting glucose levels, the FDG circulation time, and the injected FDG dose were compared using ANOVA. Multivariate regression analyses were performed to identify the potential impact of all variables described on the arterial and blood-pool FDG uptake.
Results
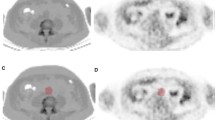
Tertile analyses revealed FDG circulation times of about 2.5 h and prescan glucose levels of less than 7.0 mmol/l, showing a favorable relationship between arterial and blood-pool FDG uptake. FDG circulation times showed negative associations with aortic meanSUVmax values as well as SVC and JV FDG blood-pool activity, but positive correlations with aortic and carotid meanTBRmax values. Prescan glucose levels were negatively associated with aortic and carotid meanTBRmax and carotid meanSUVmax values, but were positively correlated with SVC blood-pool uptake. The injected FDG dose failed to show any significant association with vascular FDG uptake.
Conclusion
FDG circulation times and prescan blood glucose levels significantly affect FDG uptake in the aortic and carotid walls and may bias the results of image interpretation in patients undergoing vascular FDG PET/CT. The injected FDG dose was less critical. Therefore, circulation times of about 2.5 h and prescan glucose levels less than 7.0 mmol/l should be preferred in this setting.



Similar content being viewed by others
References
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11.
Rudd JHF, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1009–16.
Elkhawad M, Rudd JHF. Radiotracer imaging of atherosclerotic plaque biology. Cardiol Clin. 2009;27:345–54.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24.
Sheikinea Y, Akramc K. FDG-PET imaging of atherosclerosis: do we know what we see? Atherosclerosis. 2010;211:371–80.
Camici PG, Rimoldi OE, Gaemperli O, Libby P. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012;33:1309–17.
Menezes LJ, Kotze CW, Hutton BF, Endozo R, Dickson JC, Cullum I, et al. Vascular inflammation imaging with 18F-FDG PET/CT: when to image? J Nucl Med. 2009;50:854–7.
Rudd JH, Machac J, Fayad ZA. Simvastatin and plaque inflammation. J Am Coll Cardiol. 2007;49:1991; Author reply 1991–2.
Rudd JH, Fayad ZA, Machac J, Weissberg PL, Davies JR, Warburton EA, et al. Response to ‘Laurberg JM, Olsen AK, Hansen SB, et al. Imaging of vulnerable atherosclerotic plaques with FDG-microPET: no FDG accumulation’ [Atherosclerosis 2006]. Atherosclerosis. 2007;192:453–4; Author reply 451–2.
Davies JR, Rudd JH, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, et al. Identification of culprit lesions after transient ischemic attack by combined 18F-fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke. 2005;36:2642–7.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–31.
Rudd JHF, Elkhawad M, Fayad ZA. Vascular imaging with 18F-FDG PET/CT: optimal 18F-FDG circulation time? J Nucl Med. 2009;50:1560.
Rabkin Z, Israel O, Keidar Z. Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A comparative analysis. J Nucl Med. 2010;51:1015–20.
Zhuang HM, Cortés-Blanco A, Pourdehnad M, Adam LE, Yamamoto AJ, Martínez-Lázaro R, et al. Do high glucose levels have differential effect on FDG uptake in inflammatory and malignant disorders? Nucl Med Commun. 2001;22:1123–8.
Lee WW, Chung JH, Jang SJ, Eo JS, Park SY, Sung SW, et al. Consideration of serum glucose levels during malignant mediastinal lymph node detection in non-small-cell lung cancer by FDG-PET. J Surg Oncol. 2006;94:607–13.
Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643–7.
Roy FN, Beaulieu S, Boucher L, Bourdeau I, Cohade C. Impact of intravenous insulin on 18F-FDG PET in diabetic cancer patients. J Nucl Med. 2009;50:178–83.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer: a PET study. J Nucl Med. 1993;34:1–6.
Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JH, Calcagno C, et al. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG-PET and CT imaging study. JACC Cardiovasc Imaging. 2011;4:1195–205.
Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–6.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–8.
Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301.
Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–23.
Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T; dal-PLAQUE Investigators, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–59.
Fayad ZA, Mani V, Woodward M, Kallend D, Bansilal S, Pozza JM, et al. Rationale and design of dal-PLAQUE: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Am Heart J. 2011;162:214–21.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–20.
Shepherd PR, Kahn BB. Glucose transporters and insulin action – implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–57.
Deichen JT, Prante O, Gack M, Schmiedehause K, Kuwert T. Uptake of [(18)F]fluorodeoxyglucose in human monocyte-macrophages in vitro. Eur J Nucl Med Mol Imaging. 2003;30:267–73.
Zhao S, Kuge Y, Tsukamoto E, et al. Effects of insulin and glucose loading on FDG uptake in experimental malignant tumours and inflammatory lesions. Eur J Nucl Med. 2001;28:730–5.
Zhao S, Kuge Y, Tsukamoto E, Mochizuki T, Kato T, Hikosaka K, et al. Fluorodeoxyglucose uptake and glucose transporter expression in experimental inflammatory lesions and malignant tumours: effects of insulin and glucose loading. Nucl Med Commun. 2002;23:545–50.
Bucerius J, Mani V, Moncrieff C, Rudd JH, Machac J, Fuster V, et al. Impact of noninsulin-dependent type 2 diabetes on carotid wall (18)f-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol. 2012;59:2080–8.
Silvera SS, Aidi HE, Rudd JH, Mani V, Yang L, Farkouh M, et al. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009;207:139–43.
Acknowledgment
The authors wish to thank Ash Rafique, RT, BS, CNMT, for his assistance with image acquisition. This work was partly supported by the NIHR Cambridge Biomedical Research Centre (J.H.F.R.). Partial support was provided by: NIH/NHLBI R01 HL071021 (Z.A.F.), NIH/NHLBI R01 HL078667 (Z.A.F and M.E.F.) and NIH/NCATS CTSA UL1TR000067 [Imaging Core] (Z.A.F.).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
J.H.F.R and Z.A.F contributed equally to this work and are joint senior authors.
Appendix
Appendix
Relationship between the classified prescan glucose values and arterial FDG uptake in the aorta and carotids. The FDG uptake is given as mean SUV (meanSUVmax). One-way ANOVA was performed to test for between-group differences. In the aorta, p = 0.216 for meanSUVmax; in the carotids, p = 0.170 for meanSUVmax. The data are presented as means ± standard deviation
Relationship between injected FDG dose tertiles and arterial FDG uptake in the aorta and carotids. The FDG uptake is given as mean SUV (meanSUVmax). One-way ANOVA was performed to test for between-group differences. In the aorta, p = 0.166 for meanSUVmax; in the carotids, p = 0.229 for meanSUVmax. The data are presented as means ± standard deviation
Relationship between FDG circulation time tertiles for the chest and neck scans and FDG uptake in the aorta and carotids. The FDG uptake is given as mean SUV (meanSUVmax). A one-way ANOVA was performed to test for between-group differences. In the aorta, p = 0.031 for meanSUVmax, and p = 0.034 for ≥ 78 – ≤111 min vs. >111 – < 145 min; in the carotids, p = 0.524 for meanSUVmax. The data are presented as means ± standard deviation
Rights and permissions
About this article
Cite this article
Bucerius, J., Mani, V., Moncrieff, C. et al. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur J Nucl Med Mol Imaging 41, 369–383 (2014). https://doi.org/10.1007/s00259-013-2569-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2569-6