Abstract
Purpose
The role of oxidative stress is increasingly recognized in cognitive disorders of the elderly, notably Alzheimer’s disease (AD). In these subjects brain18F-FDG PET is regarded as a reliable biomarker of neurodegeneration. We hypothesized that oxidative stress could play a role in impairing brain glucose utilization in elderly subjects with increasing severity of cognitive disturbance.
Methods
The study group comprised 85 subjects with cognitive disturbance of increasing degrees of severity including 23 subjects with subjective cognitive impairment (SCI), 28 patients with mild cognitive impairment and 34 patients with mild AD. In all subjects brain FDG PET was performed and plasma activities of extracellular superoxide dismutase (eSOD), catalase and glutathione peroxidase were measured. Voxel-based analysis (SPM8) was used to compare FDG PET between groups and to evaluate correlations between plasma antioxidants and glucose metabolism in the whole group of subjects, correcting for age and Mini-Mental State Examination score.
Results
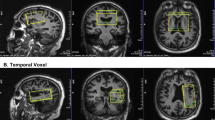
Brain glucose metabolism progressively decreased in the bilateral posterior temporoparietal and cingulate cortices across the three groups, from SCI to mild AD. eSOD activity was positively correlated with glucose metabolism in a large area of the left temporal lobe including the superior, middle and inferior temporal gyri and the fusiform gyrus.
Conclusion
These results suggest a role of oxidative stress in the impairment of glucose utilization in the left temporal lobe structures in elderly patients with cognitive abnormalities, including AD and conditions predisposing to AD. Further studies exploring the oxidative stress–energy metabolism axis are considered worthwhile in larger groups of these patients in order to identify pivotal pathophysiological mechanisms and innovative therapeutic opportunities.




Similar content being viewed by others
References
Butterfield DA, Swomley AM, Sultana R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013;19:823–35.
Smith MA, Nunomura A, Lee HG, Zhu X, Moreira PI, Avila J, et al. Chronological primacy of oxidative stress in Alzheimer’s disease. Neurobiol Aging. 2005;26:579–80.
Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26.
Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–606.
Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469:6–10.
Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617–31.
Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, et al. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–36.
Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–95.
Butterfield DA, Griffin S, Munch G, Pasinetti G. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. J Alzheimers Dis. 2002;4:193–201.
Behl C. Oxidative stress in Alzheimer’s disease: implications for prevention and therapy. Subcell Biochem. 2005;38:65–78.
Gòmez-Ramos A, Dìaz-Nido J, Smith MA, Perry G, Avila J. Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. J Neurosci Res. 2003;71:863–70.
Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:880–8.
Ames BN. Delaying the mitochondrial decay of aging. Ann N Y Acad Sci. 2004;1019:406–11.
Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75.
Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87.
Massaad CA, Pautler RG, Klann E. Mitochondrial superoxide: a key player in Alzheimer’s disease. Aging (Albany NY). 2005;1:758–61.
Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300:H1566–82.
Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. European task force on age-related white matter changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22.
Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30.
Lawton MP, Brody EM. Assessment of older people; self maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack Jr CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Loeb C, Gandolfo C. Diagnostic evaluation of degenerative and vascular dementia. Stroke. 1983;14:399–401.
Rey A. L’examenclinique en psychologie. Paris: Presses Universitaires de France; 1958.
Novelli G, Papagno C, Capitani E, Laiacona M, Cappa SF. Tre test clinici di memoria verbale a lungo termine: taratura su soggetti normali. Arch Psicol Neurol Psichiatria. 2013;47:278–96.
Carlesimo GA, Caltagirone C, Gainotti G. The mental deterioration battery: normative data, diagnostic reliability and qualitative analysis of cognitive impairment. The group for the standardization of the mental deterioration battery. Eur Neurol. 1996;36:378–84.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94.
L’Abbé MR, Fisher PWF. Automated assay of superoxide dismutase in blood. Methods Enzymol. 1990;186:232–7.
Sandström J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–6.
Zhou LC, Xiang W, Potts J, Floyd M, Sharan C, Yang H, et al. Reduction in extracellular superoxide dismutase activity in African-American patients with hypertension. Free Radic Biol Med. 2006;41:1384–91.
Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40.
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10.
Morbelli S, Drzezga A, Perneczky R, Frisoni GB, Caroli A, Van Berckel BN, et al. Resting metabolic connectivity in prodromal Alzheimer’s disease. A European Alzheimer Disease Consortium (EADC) project. Neurobiol Aging. 2012;33:2533–50.
Gispert JD, Pascau J, Reig S, Martínez-Lázaro R, Molina V, García-Barreno P, et al. Influence of the normalization template on the outcome of statistical parametric mapping of PET scans. Neuroimage. 2003;19:601–12.
Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–15.
Rodriguez G, Morbelli S, Brugnolo A, Calvini P, Girtler N, Piccardo A, et al. Global cognitive impairment should be taken into account in SPECT-neuropsychology correlations: the example of verbal memory in very mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2005;32:1186–92.
Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49.
Tasaki H, Yamashita K, Tsutsui M, Kamezaki F, Kubara T, Tanaka S, et al. Heparin-released extracellular superoxide dismutase is reduced in patients with coronary artery atherosclerosis. Atherosclerosis. 2006;187:131–8.
De la Torre JC. Alzheimer’s disease: how does it start? J Alzheimers Dis. 2002;4:497–512.
Polidori MC, Pientka L. Bridging the pathophysiology of Alzheimer’s disease with vascular pathology: the feed-back, the feed-forward, and oxidative stress. J Alzheimers Dis. 2012;28:1–9.
Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–9.
Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke. 2006;37:1850–5.
Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol. 2001;281:H1697–703.
Yada T, Shimokawa H, Morikawa K, Takaki A, Shinozaki Y, Mori H, et al. Role of Cu, Zn-SOD in the synthesis of endogenous vasodilator hydrogen peroxide during reactive hyperemia in mouse mesenteric microcirculation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H441–8.
Demchenko IT, Gutsaeva DR, Moskvin AN, Zhilyaev SY. Involvement of extracellular superoxide dismutase in regulating brain blood flow. Neurosci Behav Physiol. 2010;40:173–8.
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322:254–62.
Rajagopalan P, Toga AW, Jack CR, Weiner MW, Thompson PM. Fat-mass-related hormone, plasma leptin, predicts brain volumes in the elderly. Neuroreport. 2013;24:58–62.
Toledo JB, Da X, Bhatt P, Wolk DA, Arnold SE, Shaw LM. Relationship between plasma analytes and SPARE-AD defined brain atrophy patterns in ADNI. PLoS One. 2013;8:e55531.
den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, et al. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–5.
Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology. Brain Pathol. 2010;20:222–33.
Limón ID, Díaz A, Mendieta L, Chamorro G, Espinosa B, Zenteno E, et al. Amyloid-beta(25-35) impairs memory and increases NO in the temporal cortex of rats. Neurosci Res. 2009;63:129–37.
Wang J, Xiong S, Xie C, Markesbery WR. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–62.
Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J Bioenerg Biomembr. 2009;41:441–6.
Hovorkova P, Kristofikova Z, Horinek A, Ripova D, Majer E, Zach P, et al. Lateralization of 17beta-hydroxysteroid dehydrogenase type 10 in hippocampi of demented and psychotic people. Dement Geriatr Cogn Disord. 2008;26:193–8.
Waldbaum S, Patel M. Mitochondrial oxidative stress in temporal lobe epilepsy. Epilepsy Res. 2010;88:23–45.
Morbelli S, Piccardo A, Villavecchia G, Dessi B, Brugnolo A, Piccini A, et al. Mapping brain morphological and functional conversion patterns in amnestic MCI: a voxel-based MRI and FDG-PET study. Eur J Nucl Med Mol Imaging. 2010;37:36–45.
Bozzali M, Padovani A, Caltagirone C, Borroni B. Regional grey matter loss and brain disconnection across Alzheimer disease evolution. Curr Med Chem. 2011;18:2452–8.
Bozoki AC, Korolev IO, Davis NC, Hoisington LA, Berger KL. Disruption of limbic white matter pathways in mild cognitive impairment and Alzheimer’s disease: a DTI/FDG-PET study. Hum Brain Mapp. 2012;33:1792–802.
Grimmer T, Faust M, Auer F, Alexopoulos P, Förstl H, Henriksen G, et al. White matter hyperintensities predict amyloid increase in Alzheimer’s disease. Neurobiol Aging. 2012;33:2766–73.
Clausen A, Xu X, Bi X, Baudry M. Effects of the superoxide dismutase/catalase mimetic EUK-207 in a mouse model of Alzheimer’s disease: protection against and interruption of progression of amyloid and tau pathology and cognitive decline. J Alzheimers Dis. 2012;30:183–208.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Picco, A., Polidori, M.C., Ferrara, M. et al. Plasma antioxidants and brain glucose metabolism in elderly subjects with cognitive complaints. Eur J Nucl Med Mol Imaging 41, 764–775 (2014). https://doi.org/10.1007/s00259-013-2638-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2638-x