Abstract
Purpose
Our aim was to determine dual-phase 18F-FDG PET imaging features for various subtypes of thymic epithelial tumors based on the World Health Organization classification.
Methods
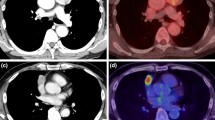
Forty-six patients with histologically verified thymic epithelial tumors [23 with low-risk tumors (4 with type A, 16 with AB, and 3 with B1) and 23 with high-risk tumors (7 with B2, 5 with B3, and 11 with thymic carcinoma] were enrolled in this study. All patients were injected with 18F-FDG.; after 1 h, they underwent scanning; after 3 h, 23 patients underwent an additional scanning. The maximum standard uptake value (SUVmax) and the retention index (RI%) of the lesions were determined.
Results
The early and delayed SUVmax values in the patients with high-risk tumors [early SUVmax (mean: 6.0) and delayed SUVmax (mean: 7.4)] were both significantly larger than those in patients with low-risk tumors [early SUVmax (mean: 3.2) and delayed SUVmax (mean: 3.4)] (P < 0.05). Early SUVmax values of greater than 7.1 differentiated thymic carcinomas from other types of tumors. For the histological differentiation between high-risk tumors and low-risk tumors, an early SUVmax value of 4.5 was used as the cutoff. The sensitivity, specificity, and accuracy were 78.3, 91.3, and 84.8%, respectively.
Conclusion
High SUV values (early SUV > 4.5) suggest the presence of high-risk tumors. A very high SUV value (early SUV > 7.1) is useful for the differentiation of thymic carcinomas from other types of tumors. The delayed SUV values were higher than the early SUV values in all types of tumors.





Similar content being viewed by others
References
Rosai J, Sobin LH. Histological typing of tumours of the thymus: international histological classification of tumours. 2nd ed. New York: Springer; 1999.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization classification of tumors: Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004.
Okumura M, Ohta M, Tateyama H, Nakagawa K, Matsumura A, Maeda H, et al. The World Health Organization histologic classification system reflects the oncological behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624–32.
Chen G, Marx A, Wen-Hu C, Yong J, Puppe B, Stroebel P, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420–9.
Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485–92.
Okumura M, Miyoshi S, Fujii Y, Takeuchi Y, Shiono H, Inoue M, et al. Clinical and functional significance of WHO classification on human thymic epithelial neoplasms: a study of consecutive 146 tumors. Am J Surg Pathol 2001;25:103–10.
Tomiyama N, Müller NL, Ellis SJ, Cleverley JR, Okumura M, Miyoshi S, et al. Invasive and non-invasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001;25:388–93.
Jung KJ, Lee KS, Han J, Kim J, Kim TS, Kim EA. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol 2001;176:433–9.
Tomiyama N, Johkoh T, Mihara N, Honda O, Kozuka T, Koyama M, et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol 2002;179:881–6.
Han J, Lee KS, Yi CA, Kim TS, Shim YA, Kim J, et al. Thymic epithelial tumors classified according to a newly established WHO scheme: CT and MR findings. Korean J Radiol 2003;4:46–53.
Jeong YJ, Lee KS, Kim J, Shim YM, Jan J, Kwon OJ. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis. AJR Am J Roentgenol 2004;183:283–9.
Inoue A, Tomiyama N, Fujimoto K, Sadohara J, Nakamichi I, Tomita Y, et al. MR imaging of thymic epithelial tumors: correlation with World Health Organization classification. Radiat Med 2006;24:171–81.
Smith CS, Schöder H, Yeung HW. Thymic extension in the superior mediastinum in patients with thymic hyperplasia: potential cause of false-positive findings on 18F-FDG PET/CT. AJR Am J Roentgenol 2007;188:1716–21.
Ferdinand B, Gupta P, Kramer EL. Spectrum of thymic uptake at 18F-FDG PET. Radiographics 2004;24:1611–6.
Patel PM, Alibazoglu H, Ali A, Fordham E, LaMonica G. Normal thymic uptake of FDG on PET imaging. Clin Nucl Med 1996;21:772–5.
Sasaki M, Kuwabara Y, Ichiya Y, Akashi Y, Yoshida T, Nakagawa M, et al. Differential diagnosis of thymic tumors using a combination of 11C-methionine PET and FDG PET. J Nucl Med 1999;40:1595–601.
Sung YM, Lee KS, Kim BT, Choi JY, Shim YM, Yi CA. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med 2006;47:1628–34.
Minn H, Clavo AC, Grénman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose and L-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med 1995;36:252–8.
Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, et al. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med 2001;28:696–703.
Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 2001;42:1412–7.
Demura Y, Tsuchida T, Ishizaki T, Mizuno S, Totani Y, Ameshima S, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med 2003;44:540–8.
Acknowledgements
This paper has not been presented, no portion of the paper has been published, and the manuscript is not under consideration for publication by any other journal.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, A., Tomiyama, N., Tatsumi, M. et al. 18F-FDG PET for the evaluation of thymic epithelial tumors: Correlation with the World Health Organization classification in addition to dual-time-point imaging. Eur J Nucl Med Mol Imaging 36, 1219–1225 (2009). https://doi.org/10.1007/s00259-009-1082-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1082-4