Abstract
Background
Patients with advanced or recurrent thymic epithelial tumors (TETs) often need several consecutive lines of chemotherapy. The aim of this retrospective monocentric study was to test whether 18F-Fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) is able to monitor standard chemotherapy efficacy in those patients and whether metabolic response correlates with morphovolumetric response as assessed by Response Evaluation Criteria in Solid Tumor (RECIST).
Methods
We evaluated 27 consecutive patients with advanced (16 patients) or recurrent (11 patients) TETs. All patients underwent 18F-FDG PET-CT before and after at least 3 cycles of chemotherapy. Maximum standardized uptake value (SUVmax) of all detected lesions was recorded and the most 18F-FDG avid lesion in each patient was selected for determination of percentage change of SUVmax (ΔSUVmax) in pre- and post-treatment scans. Tumor response was assessed by contrast-enhanced computed tomography (CE-CT) using RECIST criteria. Receiver operating characteristic (ROC) curve analysis was performed to define the optimal threshold of ΔSUVmax discriminating responders from non-responders.
Results
Metabolic response expressed as ΔSUVmax was significantly correlated with morphovolumetric response (Spearman’s rank correlation, r = 0.64, p = 0.001). ROC curve analysis showed that a ΔSUVmax value of -25% could discriminate responders from non-responders with a sensitivity of 88% and a specificity of 80%. Conversely, basal SUVmax values were not predictive of morphovolumetric tumor response.
Conclusions
Our findings indicate that metabolic response assessed by 18F-FDG PET-CT, through evaluation of ΔSUVmax, may allow identification of responders and non-responders thus guiding adaptation of therapy in patients with advanced or recurrent TETs.
Similar content being viewed by others
Background
Thymic epithelial tumors (TETs) are rare malignancies arising in the anterior mediastinum showing a high variable biological behaviour, from slow-growing benign lesions to highly aggressive carcinomas [1, 2]. According to the histological classification of the World Health Organization (WHO), TETs are subdivided into type A, AB, B1, B2, B3 and thymic carcinomas, characterized by an increasing degree of malignancy [1]. Thymic epithelial tumors are routinely staged according to Masaoka-Koga staging system, that considers the integrity of the thymic capsule (stage I), the micro or macroscopic invasion of surrounding tissues and organs (stage II and III), the presence of pleural or pericardial metastasis (stage IVA) and the lymphogenous or haematogenous metastatic spread (stage IVB) [3, 4]. Although both WHO classification and Masaoka-Koga staging system contribute to risk stratification of patients with TETs, therapeutic decisions are essentially taken on the basis of disease stage [5, 6] since WHO classification appeared to have a limited clinical predictive value [7].
The treatment strategy for thymic epithelial tumour is primarily based on whether the tumor can be radically resected or not at diagnosis [8–10]. Although surgery remains the treatment of choice, most of these tumors are unresectable or in advanced stages at diagnosis and require chemotherapy, eventually followed by surgery if tumors become resectable after the planned regimen. Furthermore, despite radical resection, recurrence is quite common in those patients and, although recurrent lesions are managed with the same approach used for newly diagnosed TETs, multicourse therapy is often necessary [8–10]. Cisplatin-based combination regimens are usually administered to patients candidate for both neoadjuvant and palliative chemotherapy [11–14]. Several consecutive lines of chemotherapy are also available for patients presenting tumor progression.
In this clinical context imaging modalities are of primary importance in the assessment of tumor resectability and for the evaluation of tumor response to chemotherapy. Contrast-enhanced computed tomography (CE-CT) is the routinely used imaging modality for diagnosis, staging and follow-up of TETs [15–19]. Furthermore in patients with advanced disease undergoing primary or definitive chemotherapy, CE-CT is usually performed to reassess resectability or to determine tumor response using Response Evaluation Criteria in Solid Tumor (RECIST) [20, 21].
Functional imaging with 18F-Fluorodeoxyglucose positron emission tomography (18F-FDG PET) with its ability to identify more aggressive and invasive subtypes of TETs provides useful information for the biologically characterization of thymic masses [22–25] and for disease stage [26–29]. Furthermore, 18F-FDG PET-CT has been performed to monitor the efficacy of targeted therapy in patients with advanced TETs and a reduction of 18F-FDG uptake higher than 30% closely correlated with objective tumor response [30]. Despite the wide use of 18F-FDG PET-CT in the assessment of metabolic response to standard chemotherapy in many solid tumors [31, 32], only few studies tested the ability of 18F-FDG PET-CT to identify responders and non-responders to standard chemotherapy in small series of patients with TETs [33–36]. Since metabolic response usually precedes the morphovolumetric reduction of tumor burden, the early detection of treatment failure may indicate the need to adopt alternative regimens [32, 37, 38]. The aim of the present study is to test whether 18F-FDG PET-CT performed in patients with advanced or recurrent TETs before and after standard chemotherapy may discriminate responders from non-responders and whether metabolic response correlates with morphovolumetric RECIST criteria of tumor response.
Methods
Patients and treatment
In this retrospective monocentric study we evaluated the medical records of twenty-seven consecutive patients, 18 male (mean age ± SD, 56 ± 12 y) and 9 female (mean age ± SD, 57 ± 11 y), with advanced (16 patients) or recurrent (11 patients) thymic epithelial tumors who had undergone whole-body 18F-FDG PET-CT before and after standard chemotherapy regimens. Histopathogical diagnosis was obtained in all patients and, based on WHO classification, 1 B1, 7 B2 and 7 B3 TETs along with 12 thymic carcinoma were included in the study. All patients were staged using the Masaoka-Koga staging system based on CE-CT findings at presentation: 3 patients had unresectable stage III, 5 were in stage IVA and 19 patients had stage IVB which included a high percentage of thymic carcinomas (11 patients).
Among the 27 patients, 16 had no chemotherapy before the basal 18F-FDG PET-CT whereas 5 patients received prior adjuvant or neoadjuvant chemotherapy and 6 were treated with definite chemotherapy for advanced, unresectable TET (Table 1). Furthermore, 5 patients underwent radiation therapy after surgery or in combination with chemotherapy. After the basal 18F-FDG PET-CT scan, platinum-based chemotherapy was administered to 23 patients (3-8 cycles, median 5); four additional patients with advanced disease who were in progression after platinum-based chemotherapy were treated with gemcitabine-capecitabine (at least 7 cycles).
Response evaluation
Contrast-enhanced CT scan of skull, neck, chest, abdomen and pelvis was performed at baseline and at the end of the planned regimen and the effects of chemotherapy were assessed using the RECIST version 1.1 [21]. Tumor response was defined as: complete response (CR) when there was disappearance of all lesions; partial response (PR) if there was ≥ 30% reduction in lesion size; progressive disease (PD) if there was increase of more than 20% in lesion size or appearance of a new lesion; stable disease (SD) when no PR and no PD occurred. For statistical purposes patients with CR and PR were grouped in the class of responders whereas patients with SD and PD were considered non-responders.
18F-FDG PET-CT Study
18F-FDG PET-CT scans were acquired after fasting for 8 h and 60 min after intravenous administration of 18F-FDG (350–370 MBq). The blood glucose level, measured just before tracer administration, was < 120 mg/dL in all patients. Dual-modality imaging was performed with a PET-CT Discovery LS scanner (GE Healthcare, Milan, Italy) consisting of a PET scanner and a four-row multidetector computed tomography (MDCT) system. MDCT scan was acquired using the following parameters: 4 × 5 mm collimation (140 kV, 80 mAs), 0.8 s rotation time, pitch of 1.5; when indicated, a fully diagnostic contrast-enhanced CT was performed. PET scan was subsequently performed in 2-dimensional mode using 4 min per bed position and six to eight bed positions per patient, depending on patient height. Iterative images reconstruction was completed with an ordered subsets-expectation maximization algorithm (2 iterations, 28 subsets). Attenuation corrected emission data were obtained using filtered back projection CT reconstructed images (Gaussian filter with 8 mm full width half maximum) to match the PET resolution. Transaxial, sagittal, and coronal images as well as coregistered images were examined using Xeleris software and then transferred in DICOM format to an OsiriX workstation (Pixmeo, Switzerland). All areas of focal 18F-FDG uptake visible on 2 contiguous PET slices at least and not corresponding to physiological tracer uptake were considered to be positive [39]. The SUVmax values of all lesions in the pre- and post-treatment scan were recorded by two board-certified nuclear medicine physicians and discrepancies between their assessments were resolved by consensus through discussion. The SUVmax value of the most metabolically active lesion in each examination was used to define the ΔSUVmax as follows: ΔSUVmax = [(SUVmax post – SUVmax pre)/SUVmax pre] × 100.
Statistical analysis
Statistical analysis was performed using the software MedCalc for Windows, version 10.3.2.0, (MedCalc Software, Mariakerke, Belgium). Data are expressed as mean ± SD if not differently indicated. Unpaired Student’s t test was used to compare means of normally distributed data sets as assessed by Kolmogorov-Smirnov test. Spearman’s rank correlation was used to examine the association between ∆SUVmax and tumor response. Receiver operating characteristic (ROC) curve analysis was performed to estimate the best value of ∆SUVmax capable of discriminating responders from non-responders. A p value < 0.05 was considered statistically significant.
Results
Patient characteristics are summarized in Table 1. Pre-treatment 18F-FDG PET-CT scan showed abnormal 18F-FDG uptake in all patients detecting a total of 77 lesions, including 18 mediastinal masses, 15 lymph nodes, 23 pleura/pericardial implants, 16 visceral lesions and 5 bone lesions, with an average of 2.85 ± 2.03 lesions per patient (range 1-8). The lesion with the highest SUVmax value in each patient was selected as the target lesion for the assessment of metabolic response; these 27 target lesions showed a mean size of 52.90 mm ± 21.24 mm. The SUVmax values of those lesions ranged between 3.3 (pleural implant) and 20 (thymic carcinoma) with a mean of 8.67 ± 4.89 (Table 2). Post-treatment 18F-FDG PET-CT showed reduction of FDG uptake in the target lesion of 19 patients and an increase of tracer accumulation in 8 patients (Table 2). None of the patients showing reduction of 18F-FDG in the target lesion showed the appearance of new site of abnormal 18F-FDG uptake whereas new metabolically active lesions (3 metastatic lymph nodes, 1 lung lesion, 3 pleural implants and 1 large vessel infiltration) were found in 5 out of 8 patients showing increased 18F-FDG accumulation in the target lesion.
After treatment with standard chemotherapy, morphovolumetric tumor response was assessed by contrast-enhanced CT. Based on RECIST criteria, an objective tumor response was observed in 17 patients (2 CR and 15 PR) whereas in the remaining patients, 8 showed SD and 2 had PD (Table 2).
Normally distributed SUVmax values of pre- and post-treatment 18F-FDG PET-CT scan in responders and non-responders were expressed as mean ± SD and compared. SUVmax values of pre-treatment 18F-FDG PET-CT scan were not significantly different between responders and non-responders (8.80 ± 5.04 vs 8.45 ± 4.88, p = 0.8645). Conversely SUVmax values of post-treatment 18F-FDG PET-CT scan were significantly lower in responders as compared to non-responders (3.94 ± 3.62 vs 8.99 ± 4.34, p = 0.0038).
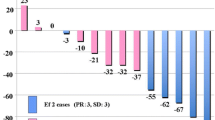
The change of 18F-FDG uptake between baseline and post-treatment scan was -46.82% ± 8.10% (SE) in responders, indicating a reduction of tracer uptake, whereas non-responders showed an increase of 18F-FDG uptake with a ΔSUVmax of 20.40% ± 15.75% (SE). The normally distributed values of ΔSUVmax were significantly different in responders and non-responders (p = 0.0003, unpaired t-test) and were significantly correlated with morphovolumetric response (Spearman’s rank correlation, r = 0.64, p = 0.001). Fig. 1 shows the distribution of ΔSUVmax values in responders and non-responders.
Distribution of ΔSUVmax values in patients allocated in the class of responders and non-responders by RECIST criteria. Responders showed ΔSUVmax values significantly lower than those of non-responders (p = 0.0003, unpaired t-test) and a significant correlation was found between ΔSUVmax values and morphovolumetric response (Spearman’s rank correlation, r = 0.64, p = 0.001). Horizontal bar indicates mean
ROC curve analysis showed that a ΔSUVmax value of -25% could discriminate responders from non-responders with a sensitivity of 88% and a specificity of 80% (Fig. 2).
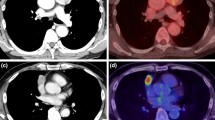
Figures 3 and 4 show representative 18F-FDG PET-CT images of baseline and post-treatment scans in a patient with metabolic response and a patient with metabolic progression of the disease, respectively. The responding patient of Fig. 3 was allocated in the class of PR using RECIST and showed a 47% reduction of tracer uptake indicating a concordance between morphologic and metabolic tumor response. Conversely the non-responding patient of Fig. 4 was judged to have stable disease by RECIST but he showed a 64% increase of 18F-FDG uptake indicating a metabolic progression.
Representative images of baseline a and post-treatment b 18F-FDG PET-CT scan in a patient with thymic carcinoma. Fusion images of co-registered transaxial 18F-FDG PET and contrast-enhanced CT sections are shown. In the baseline scan SUVmax was 6.60 whereas the post-treatment study showed a SUVmax of 3.50. A 47% reduction of 18F-FDG uptake was found in this patient with partial response based on RECIST. The same maximum threshold of SUV was applied to PET images from pre-treatment and post-treatment scans as shown by the color scale on the left
Representative images of baseline a and post-treatment b 18F-FDG PET-CT scan in a patient with thymic carcinoma. Fusion images of co-registered transaxial 18F-FDG PET and CT sections are shown. In the baseline scan SUVmax was 8.80 whereas the post-treatment study showed a SUVmax of 14.40. A 64% increase of 18F-FDG uptake was found in this patient with stable disease based on RECIST. The same maximum threshold of SUV was applied to PET images from pre-treatment and post-treatment scans as shown by the color scale on the left
Discussion
The present study showed that 18F-FDG PET-CT may be used to monitor tumor response to standard chemotherapy in patients with advanced or recurrent TETs. The percentage change of 18F-FDG uptake between baseline and post-treatment scans was indeed able to discriminate responders from non-responders and significantly correlated with tumor response assessed by RECIST criteria. In particular, a 25% reduction of 18F-FDG uptake identified responders with a sensitivity of 88% and a specificity of 80%. Our findings are in agreement with previous studies evaluating early metabolic response in patients with TETs mainly treated with targeted therapy [30]. All patients in our study received conventional chemotherapy for advanced or recurrent disease and, being potentially candidate to several consecutive lines of chemotherapy, tumor response was carefully assessed to guide subsequent therapeutic options.
Assessment of tumor response in patients with TETs is usually performed using RECIST criteria in which unidimensional tumor measurements are obtained from pre-and post-treatment CT scans to evaluate changes of tumor burden in response to therapy [20, 21]. Although RECIST criteria are widely accepted as the standard method to evaluate tumor response in solid tumors, they have some limitations in TETs. In fact, TETs differ from other solid tumors in terms of growth and dissemination patterns especially in advanced stages. They are often large masses with indefinite borders encasing mediastinal structures and infiltrating adjacent tissues. Furthermore non-contiguous pleural metastases are common in these patients and measurements of these lenticular lesions may be difficult. In order to overcome these limitations, International Thymic Malignancy Interest Group proposed modified RECIST criteria for the assessment of tumor response in TETs taking into account the peculiar growth and dissemination patterns of the disease [40–44].
Although standard criteria for the assessment of objective tumor response remain based on anatomical measurements, functional imaging with 18F-FDG-PET-CT has been used for the evaluation of metabolic response to therapy in many solid tumors. Previous studies showed indeed that conventional cytotoxic agents, by inducing tumor cell death, cause a reduction of cell viability and glucose demand with a consequent decrease of 18F-FDG uptake that may precede tumor shrinkage as assessed by anatomical measurements [32, 37, 38, 45, 46]. Due to the consistent results of a number of studies, recommendations on the use of 18F-FDG-PET for monitoring efficacy of therapy have been published and include EORTC (European Organization for Research and Treatment of Cancer) and PERCIST (PET Response Criteria in Solid Tumors) criteria which are based on changes of tracer uptake in response to treatment [31, 47]. Although clinically relevant thresholds have been proposed to classify metabolic response [48, 49] the optimal cut-off to discriminate responders and non-responders may vary among different malignancies depending on their tracer uptake patterns and dynamics during therapy. In our study, the optimal threshold that identifies responding and non-responding TETs is in agreement with the values proposed by both EORTC and PERCIST recommendations.
Despite the large use of 18F-FDG in the evaluation of metabolic response of solid tumors to therapy, it is still not clear how many lesions should be included in the analysis of pre and post-treatment PET scans especially in advanced stages. Previous studies reported analysis of both single and multiple lesions and both approaches resulted to be predictive of morphovolumetric response or outcome [48, 50]. Considering the potential association between 18F-FDG uptake and degree of invasiveness of TETs and the possible coexistence of different WHO histotypes in the same tumor mass, we decide to analyse the most 18F-FDG avid lesion in pre and post-treatment scans in order to derive the percentage change of tracer uptake, after ensuring that no new lesions were found or metabolic progression occurred in all other lesions in post-treatment 18F-FDG PET-CT. This simplified approach may be easily employed to evaluate metabolic response in patients with TETs in daily clinical practice although we are aware that other volume-based metabolic parameters, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) may better reflect metabolic response in all lesions and be more reliable predictive markers of survival [51].
Limitations of our monocentric study are the retrospective analysis of imaging findings and the relatively limited series of patients. Therefore further studies are needed to confirm our findings in a larger population of patients and, since TETs are rare tumors, this may require the involvement of several institutions.
Conclusions
Our study showed that metabolic response assessed by 18F-FDG PET-CT may complement RECIST criteria in the identification of responders and non-responders thus providing an additional guide for adaptation of therapy in patients with advanced or recurrent thymic epithelial tumors.
Abbreviations
- 18F-FDG PET-CT:
-
18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography
- CE-CT:
-
Contrast-Enhanced Computed Tomography
- CR:
-
Complete Response
- EORTC:
-
European Organization for Research and Treatment of Cancer
- MDCT:
-
Multidetector Computed Tomography
- MTV:
-
Metabolic Tumor Volume
- PD:
-
Progressive Disease
- PERCIST:
-
PET Response Criteria in Solid Tumors
- PR:
-
Partial Response
- RECIST:
-
Response Evaluation Criteria in Solid Tumor
- ROC:
-
Receiver Operating Characteristic
- SD:
-
Stable Disease
- SUVmax :
-
Maximum Standardized Uptake Value
- TETs:
-
Thymic Epithelial Tumors
- TLG:
-
Total Lesion Glycolysis
- WHO:
-
World Health Organization
- ΔSUVmax :
-
percentage change of SUVmax in pre- and post-treatment scans.
References
Srirajaskanthan R, Toubanakis C, Dusmet M, Caplin ME. A review of thymic tumours. Lung Cancer. 2008;60:4–13.
Priola AM, Priola SM. Imaging of thymus in myasthenia gravis: from thymic hyperplasia to thymic tumor. Clin Radiol. 2014;69:e230–45.
Masaoka A. Staging system of thymoma. J Thorac Oncol. 2010;5:S304–12.
Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6:S1710–6.
Rena O, Papalia E, Maggi G, Oliaro A, Ruffini E, Filosso P, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer. 2005;50:59–66.
Strobel P, Bauer A, Puppe B, Kraushaar T, Krein A, Toyka K, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004;22:1501–9.
Benveniste MF, Rosado-de-Christenson ML, Sabloff BS, Moran CA, Swisher SG, Marom EM. Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics. 2011;31:1847–61. discussion 61-3.
Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S, Committee EG. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v40–55.
Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4:911–9.
Girard N, Mornex F, Van Houtte P, Cordier JF, van Schil P. Thymoma: a focus on current therapeutic management. J Thorac Oncol. 2009;4:119–26.
Loehrer Sr PJ, Jiroutek M, Aisner S, Aisner J, Green M, Thomas Jr CR, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer. 2001;91:2010–5.
Lemma GL, Lee JW, Aisner SC, Langer CJ, Tester WJ, Johnson DH, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29:2060–5.
Hirai F, Yamanaka T, Taguchi K, Daga H, Ono A, Tanaka K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol. 2015;26:363–8.
Palmieri G, Merola G, Federico P, Petillo L, Marino M, Lalle M, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs). Ann Oncol. 2010;21:1168–72.
Marom EM, Milito MA, Moran CA, Liu P, Correa AM, Kim ES, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol. 2011;6:1274–81.
Zhao Y, Chen H, Shi J, Fan L, Hu D, Zhao H. The correlation of morphological features of chest computed tomographic scans with clinical characteristics of thymoma. Eur J Cardiothorac Surg. 2015;48:698–704.
Tomiyama N, Honda O, Tsubamoto M, Inoue A, Sumikawa H, Kuriyama K, et al. Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol. 2009;69:280–8.
Marom EM. Advances in thymoma imaging. J Thorac Imaging. 2013;28:69–80. quiz 1-3.
Qu YJ, Liu GB, Shi HS, Liao MY, Yang GF, Tian ZX. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol. 2013;20:66–72.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
De Luca S, Fonti R, Palmieri G, Federico P, Del Prete G, Pacelli R, et al. Combined imaging with 18F-FDG-PET/CT and 111In-labeled octreotide SPECT for evaluation of thymic epithelial tumors. Clin Nucl Med. 2013;38:354–8.
Sung YM, Lee KS, Kim BT, Choi JY, Shim YM, Yi CA. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med. 2006;47:1628–34.
Endo M, Nakagawa K, Ohde Y, Okumura T, Kondo H, Igawa S, et al. Utility of 18FDG-PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer. 2008;61:350–5.
Nakajo M, Kajiya Y, Tani A, Yoneda S, Shirahama H, Higashi M, et al. (1)(8)FDG PET for grading malignancy in thymic epithelial tumors: significant differences in (1)(8)FDG uptake and expression of glucose transporter-1 and hexokinase II between low and high-risk tumors: preliminary study. Eur J Radiol. 2012;81:146–51.
Park SY, Cho A, Bae MK, Lee CY, Kim DJ, Chung KY. Value of 18F-FDG PET/CT for Predicting the World Health Organization Malignant Grade of Thymic Epithelial Tumors: Focused in Volume-Dependent Parameters. Clin Nucl Med. 2016;41:15–20.
Benveniste MF, Moran CA, Mawlawi O, Fox PS, Swisher SG, Munden RF, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol. 2013;8:502–10.
Terzi A, Bertolaccini L, Rizzardi G, Luzzi L, Bianchi A, Campione A, et al. Usefulness of 18-F FDG PET/CT in the pre-treatment evaluation of thymic epithelial neoplasms. Lung Cancer. 2011;74:239–43.
Viti A, Terzi A, Bianchi A, Bertolaccini L. Is a positron emission tomography-computed tomography scan useful in the staging of thymic epithelial neoplasms? Interact Cardiovasc Thorac Surg. 2014;19:129–34.
Thomas A, Mena E, Kurdziel K, Venzon D, Khozin S, Berman AW, et al. 18F-fluorodeoxyglucose positron emission tomography in the management of patients with thymic epithelial tumors. Clin Cancer Res. 2013;19:1487–93.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507.
Kaira K, Murakami H, Miura S, Kaira R, Akamatsu H, Kimura M, et al. 18F-FDG uptake on PET helps predict outcome and response after treatment in unresectable thymic epithelial tumors. Ann Nucl Med. 2011;25:247–53.
Kaira K, Endo M, Abe M, Nakagawa K, Ohde Y, Okumura T, et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol. 2010;28:3746–53.
Korst RJ, Bezjak A, Blackmon S, Choi N, Fidias P, Liu G, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg. 2014;147:36–44. 6 e1.
Kim HS, Lee JY, Lim SH, Sun JM, Lee SH, Ahn JS, et al. A Prospective Phase II Study of Cisplatin and Cremophor EL-Free Paclitaxel (Genexol-PM) in Patients with Unresectable Thymic Epithelial Tumors. J Thorac Oncol. 2015;10:1800–6.
Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101–11.
Weber WA, Wieder H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol Imaging. 2006;33 Suppl 1:27–37.
Fonti R, Salvatore B, Quarantelli M, Sirignano C, Segreto S, Petruzziello F, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in evaluation of patients with multiple myeloma. J Nucl Med. 2008;49:195–200.
Marom EM, Detterbeck FC. Overview. J Thorac Oncol. 2014;9:S63–4.
Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S65–72.
Nicholson AG, Detterbeck FC, Marino M, Kim J, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S73–80.
Kondo K, Van Schil P, Detterbeck FC, Okumura M, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S81–7.
Benveniste MF, Korst RJ, Rajan A, Detterbeck FC, Marom EM, International Thymic Malignancy Interest G. A practical guide from the International Thymic Malignancy Interest Group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J Thorac Oncol. 2014;9:S119–24.
Kasamon YL, Wahl RL. FDG PET and risk-adapted therapy in Hodgkin’s and non-Hodgkin’s lymphoma. Curr Opin Oncol. 2008;20:206–19.
Kasamon YL, Jones RJ, Wahl RL. Integrating PET and PET/CT into the risk-adapted therapy of lymphoma. J Nucl Med. 2007;48 Suppl 1:19S–27S.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82.
Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48:1626–32.
Ott K, Herrmann K, Lordick F, Wieder H, Weber WA, Becker K, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res. 2008;14:2012–8.
Avril N, Sassen S, Schmalfeldt B, Naehrig J, Rutke S, Weber WA, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol. 2005;23:7445–53.
Moon SH, Kim HS, Cho YS, Sun JM, Ahn JS, Park K, et al. Value of volume-based early metabolic response in patients with unresectable thymic epithelial tumor. Lung Cancer. 2016;100:24–9.
Acknowledgements
The authors would like to acknowledge AIRC, Associazione Italiana per la Ricerca sul Cancro (project No. IG-17249) and POR (Programma Operativo Regionale) Campania FESR (Fondo Europeo Sviluppo Regionale) 2007/2013, Rete delle Biotecnologie Campane.
The authors would like to thank Dr Viviana De Rosa for her help in the preparation of this manuscript and the Rare Tumors Reference Center of University of Naples Federico II for patient’s care.
Funding
This work was partly supported by AIRC, Associazione Italiana per la Ricerca sul Cancro (project No. IG-17249) and POR (Programma Operativo Regionale) Campania FESR (Fondo Europeo Sviluppo Regionale) 2007/2013, Rete delle Biotecnologie Campane.
Availability of data and materials
All relevant data generated or analysed during this study are included into the manuscript.
Authors’ contributions
SS, SDV, GP and MO were responsible for study design and conception; data collection was performed by MO, SP and VD; SS, SDV, RF and LP were responsible for data analysis and interpretation; RF and SP carried out image processing; manuscript was drafted by SS and SDV. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All images are entirely unidentifiable and there are no details on individual patients reported within the manuscript.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (Protocol N. 133/16) and utilized a retrospective analysis of anonymized imaging and clinical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Segreto, S., Fonti, R., Ottaviano, M. et al. Evaluation of metabolic response with 18F-FDG PET-CT in patients with advanced or recurrent thymic epithelial tumors. Cancer Imaging 17, 10 (2017). https://doi.org/10.1186/s40644-017-0112-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-017-0112-x