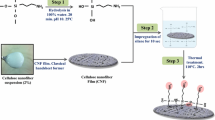
Abstract
Today, enzymatic treatment is a progressive field in combating biofilm producing pathogens. In this regard, serratiopeptidase, a medicinally important metalloprotease, has been recently highlighted as an enzyme with proved anti-biofilm activity. In the present study, in order to increase the long-lasting effects of the enzyme, serratiopeptidase and the novel engineered forms with enhanced anti-biofilm activity were immobilized on the surface of cellulose nanofibers (CNFs) as a natural polymer with eminent properties. For this, recombinant serratiopeptidases including the native and previously designed enzymes were produced, purified and conjugated to the CNF by chemical and physical methods. Immobilization was confirmed using different scanning and microscopic methods. The enzyme activity was assessed using casein hydrolysis test. Enzyme release analysis was performed using dialysis tube method. Anti-biofilm activity of free and immobilized enzymes has been examined on Staphylococcus aureus and Pseudomonas aeruginosa strains. Finally, cytotoxicity of enzyme-conjugated CNFs was performed by MTT assay. The casein hydrolysis results confirmed fixation of all recombinant enzymes on CNFs by chemical method; however, inadequate fixation of these enzymes was found using cold atmospheric plasma (CAP). The AFM, FTIR, and SEM analysis confirmed appropriate conjugation of enzymes on the surface of CNFs. Immobilization of enzymes on CNFs improved the anti-biofilm activity of serratiopeptidase enzymes. Interestingly, the novel engineered serratiopeptidase (T344 [8-339ss]) exhibited the highest anti-biofilm activity in both conjugated and non-conjugated forms. In conclusion, incorporation of serratiopeptidases into CNFs improves their anti-biofilm activities without baring any cytotoxicity.
Key points
• Enzymes were successfully immobilized on cellulose nanofibers using chemical method.
• Immobilization of enzymes on CNFs improved their anti-biofilm activity.
• T344 [8-339ss] exhibited the highest anti-biofilm activity in both conjugated and non-conjugated forms.





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon a reasonable request.
References
Ahmadi S, Seraj M, Chiani M, Hosseini S, Bazzazan S, Akbarzadeh I, Saffar S, Mostafavi E (2022) In vitro development of controlled-release nanoniosomes for improved delivery and anticancer activity of letrozole for breast cancer treatment. Int J Nanomedicine 17:6233–6255. https://doi.org/10.2147/IJN.S384085
Aishwarya SS, Selvarajan E, Iyappan S, Rajnish KN (2019) Recombinant l-asparaginase II from Lactobacillus casei subsp. casei ATCC 393 and its anticancer activity. Indian. J Microbiol 59(3):313–320. https://doi.org/10.1007/s12088-019-00806-0
Bhagat S, Agarwal M, Roy V (2013) Serratiopeptidase: a systematic review of the existing evidence. Int J Surg 11:209–217
Bhardwaj N, Kundu SC (2010) Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28:325–347
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao L (2005) Immobilised enzymes: science or art? Curr Opin Biotechnol 9:217–226
Chandrasekaran SD, Vaithilingam M, Shanker R, Kumar S, Thiyur S, Babu V, Selvakumar JN, Prakash S (2015) Exploring the in vitro thrombolytic activity of nattokinase from a new strain Pseudomonas aeruginosa CMSS. Jundishapur J Microbiol 8(10):e23567. https://doi.org/10.5812/jjm.23567
Christensen GD, Baldassarri L, Simpson WA (1994) Colonization of medical devices by coagulase-negative staphylococci. ASM Press, Washington D.C.
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay - casein as a aubstrate. J Vis Exp 19:899
Datta S, Rajnish KN, George Priya Doss C, Melvin Samuel S, Selvarajan E, Zayed H (2020) Enzyme therapy: a forerunner in catalyzing a healthy society? Expert Opin Biol Ther 20(10):1151–1174. https://doi.org/10.1080/14712598.2020.1787980
Demirkan E, Avci T, Aykut Y (2018) Protease immobilization on cellulose monoacetate/chitosan-blended nanofibers. J Ind Text 47(8):2092–2111
Esch PM, Gemgross H, Fabian A (1989) Reduction of postoperative swelling, objective measurement of swelling of the upper ankle joint in treatment with serrapeptase-a prospective study. Fortschr Med 107(4):67–68
Ethiraj S, Gopinath S (2017) Production, purification, characterization, immobilization, and application of Serrapeptase: a review. Front Biol 12(5):333–348
Gopinath S, Venkataprasad R, Rajnish KN, Datta S, Selvarajan E (2020) Enhancement of serrapeptase hyper producing mutant by combined chemical and UV mutagenesis and its potential for fibrinolytic activity. J Pure Appl Microbiol 14(2):1295–1303. https://doi.org/10.22207/JPAM.14.2.25
Hernandez K, Fernandez-Lafuente R (2011) Control of protein immobilization:coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb Technol 48:107–122
House JL, Anderson EM, Ward WK (2007) Immobilization techniques to avoid enzyme loss from oxidase-based biosensors: a one-year study. J Diabetes Sci Technol 1(1):18–27. https://doi.org/10.1177/193229680700100104
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng Life Sci 13(1):49–61
Jackson E, Correa S, Betancor L (2019) Cellulose-based nanosupports for enzyme immobilization. In: Mondal M (ed) Cellulose-based superabsorbent hydrogels, polymers and polymeric composites: a reference series. Springer, Cham
Jadav SP, Patel NH, Shah TG (2010) Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother 1(2):116–117
Jadhav SB, Shah N, Rathi A, Rathi V, Rathi A (2020) Serratiopeptidase: insights into the therapeutic applications. Biotechnol Rep 28:e00544
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821
Joshi KK, Nerurkar RP (2012) Anti-inflammatory effect of the serratiopeptidase–rationale or fashionable: a study in rat paw edema model induced by the carrageenan. Indian J Physiol Pharmacol 56(4):367–374
Kaur H, Singh A (2015) Design, development and characterization of serratiopeptidase loaded albumin nanoparticles. J Appl Pharm Sci 5:103–109
Khanna A, Khanna M, Aggarwal A (2013) Serratia marcescens-a rare opportunistic nosocomial pathogen and measures to limit its spread in hospitalized patients. J Clin Diagn Res 7(2):243–246
Kumar S, Jana AK, Dhamija I, Maiti M (2014) Chitosan-assisted immobilization of serratiopeptidase on magnetic nanoparticles, characterization and its target delivery. J Drug Target 22(2):123–137. https://doi.org/10.3109/1061186X.2013.844157
Kumar S, Jana AK, Dhamija I, Singla Y, Maiti M (2013) Preparation, characterization and targeted delivery of serratiopeptidase immobilized on amino-functionalized magnetic nanoparticles. Eur J Pharm Biopharm 85(3 Pt A):413–426
Leipner J, Saller R (2000) Systemic enzyme therapy in oncology: effect and mode of action. Drugs 59(4):769–780
Liu Y, Chen JY (2016) Enzyme immobilization on cellulose matrixes. J Bioact Compat Polym 31:553–567
Mahlen SD (2011) Serratia infections: from military experiments to current practice. Clin Microbiol Rev 24(4):755–791
Malshe PC (1998) Orally administered serratiopeptidase: can it work? J Assoc Physicians India 46(5):492
McDonough M, Perov P, Johnson W, Radojev S (2020) Data processing and analysis for atomic force microscopy (AFM) undergraduate theses and capstone projects. 18. https://dc.suffolk.edu/undergrad/18/
Mecikoglu M, Saygi B, Yildirim Y, Karadag-Saygi E, Ramadan SS, Esemenli T (2006) The effect of proteolytic enzyme serratiopeptidase in the treatment of experimental implant-related infection. J Bone Joint Surg Am 88(6):1208–1214
Metkar SK, Girigoswami A, Murugesan R, Girigoswami K (2017) In vitro and in vivo insulin amyloid degradation mediated by serratiopeptidase. Mater Sci Eng C 70:728–735
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37(5):790–802
Narasimhan MK, Ethiraj S, Krishnamurthi T, Rajesh M (2018) Purification, biochemical, and thermal properties of fibrinolytic enzyme secreted by Bacillus cereus SRM-001. Prep Biochem Biotechnol 48(1):34–42. https://doi.org/10.1080/10826068.2017.1387560
Panagariya A, Sharma AK (1999) A preliminary trial of serratiopeptidase in patients with carpal tunnel syndrome. J Assoc Physicians India 47(12):1170–1172
Panthi VK, Jha SK, Chaubey R, Pangeni R (2021) Formulation and development of serratiopeptidase enteric coated tablets and analytical method validation by UV Spectroscopy. Int J Anal Chem 2021:9749474. https://doi.org/10.1155/2021/9749474
Rajnish KN, Samuel MS, John A, Datta S, Narendhar C, Balaji R, Jose SP, Selvarajan E (2021) Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review. Int J Biol Macromol 182:1793–1802. https://doi.org/10.1016/j.ijbiomac.2021.05.176
Rouhani M, Valizadeh V, Aghai A, Pourasghar S, Molasalehi S, Cohan RA, Norouzian D (2021) Design, expression and functional assessment of novel engineered serratiopeptidase analogs with enhanced protease activity and thermal stability. World J Microbiol Biotechnol 38(1):17–17
Rouhani M, Valizadeh V, Cohan RA, Norouzian D (2018) Computational design, structure refinement and molecular dynamics simulation of novel engineered serratiopeptidase analogs. J Biomol Struct Dyn 1:11
Rouhani M, Valizadeh V, Molahsalehi S, Norouzian D (2020) Production and expression optimization of heterologous serratiopeptidase. Iran J Public Health 49:931–999
Selan L, Papa R, Tilotta M, Vrenna G, Carpentieri A, Amoresano A, Pucci P, Artini M (2015) Serratiopeptidase: a well-known metalloprotease with a new non-proteolytic activity against S. aureus biofilm. BMC Microbiol 15(1):207
Selvarajan E, Mohanasrinivasan V, Subathra Devi C, George Priya Doss C (2015) Immobilization of β-galactosidase from Lactobacillus plantarum HF571129 on ZnO nanoparticles: characterization and lactose hydrolysis. Bioprocess Biosyst Eng 38:1655–1669. https://doi.org/10.1007/s00449-015-1407-6
Sheldon RA, van Pelt S (2013) Enzyme immobilization in biocatalysis: why, what and how? Chem Soc Rev 42:6223–6225
Srinivasan V, Ak S, Mondal M, Dhar SC (2013) Screening for staphylokinase producing staphylococcus spp. from bovine milk sample. Int. J Pharm Pharm Sci 5(2):601–604
Sulaiman S, Mokhtar MN, Naim MN, Baharuddin AS, Sulaiman A (2014) A review: potential usage of cellulose nanofibers (CNF) for enzyme immobilization via covalent interactions. Appl Biochem Biotechnol 175:1817–1842
Tiwari M (2017) The role of serratiopeptidase in the resolution of inflammation. Asian J Pharm Sci 12(3):209–215
Acknowledgements
The authors wish to express their deep gratitude to all who provided support during the course of this research. Dr. Seyed Ali Nojoumi kindly revised the manuscript for proper language and grammar.
Funding
This project was financially supported by Pasteur Institute of Iran.
Author information
Authors and Affiliations
Contributions
MR, SAH, and SM contributed to the laboratory work, analysis of the data, and drafted the paper. HB, SMA, and DN offered critical suggestions for designing experimental assays and data analysis. V. V designed the work, supervised the study, and critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article contained no studies incorporating human participants or animals to be performed by any of the authors. The local Ethics Review Committee approved the study protocol at the Pasteur Institute of Iran, Tehran, Iran (approval ID: IR.PII.REC.1398.040).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rouhani, M., Valizadeh, V., Bakhshandeh, H. et al. Improved anti-biofilm activity and long-lasting effects of novel serratiopeptidase immobilized on cellulose nanofibers. Appl Microbiol Biotechnol 107, 6487–6496 (2023). https://doi.org/10.1007/s00253-023-12734-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12734-7