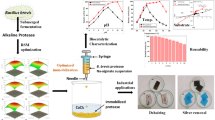
Abstract
The food industry has developed a wide range of products with reduced lactose to allow people with intolerance to consume dairy products. Although β-galactosidase has extensive applications in the food, pharma, and biotechnology industries, the enzymes are high-cost catalysts, and their use makes the process costly. Immobilization is a viable strategy for enzyme retention inside a reactor, allowing its reuse and application in continuous processes. Here, we studied the immobilization of β-galactosidase from Bacillus licheniformis in ion exchange resin. A central composite rotational design (CCRD) was proposed to evaluate the immobilization process in relation to three immobilization solution variables: offered enzyme activity, ionic strength, and pH. The conditions that maximized the response were offered enzyme activity of 953 U, 40 mM ionic strength, and pH 4.0. Subsequently, experiments were performed to provide additional stabilization for biocatalyst, using a buffer solution pH 9.0 at 25 °C for 24 h, and crosslinking with different concentrations of glutaraldehyde. The stabilization step drastically impacted the activity of the immobilized enzyme, and the reticulation with different concentrations of glutaraldehyde showed significant influence on the activity of the immobilized enzyme. In spite of substantially affecting the initial activity of the immobilized enzyme, higher reagent concentrations (3.5 g L−1) were effective for maintaining stability related to the number of cycles of the enzyme immobilized. The β-galactosidase from Bacillus licheniformis immobilized in Duolite A568 is a promising technique to produce reduced or lactose-free dairy products, as it allows reuse of the biocatalyst, decreasing operational costs.
Key Points
• Immobilization of β-galactosidase from Bacillus licheniformis in batch reactor
• Influence of buffer pH and ionic concentration and offered enzyme activity on immobilization
• Influence of glutaraldehyde on operational stability






Similar content being viewed by others
References
Ansari SA, Satar R, Zaidi SK, Naseer MI, Karim S, Alqahtani MH, Rasool M (2015) Nanodiamonds as an effective and novel matrix for immobilizing β galactosidase. Food Bioprod Process 95:298–303. https://doi.org/10.1016/j.fbp.2014.10.014
Arsalan A, Alam MF, Farheen Zofair SF, Ahmad S, Younus H (2020) Immobilization of β-galactosidase on tannic acid stabilized silver nanoparticles: a safer way towards its industrial application. Spectrochim Acta - Part A Mol Biomol Spectrosc 226:117637. https://doi.org/10.1016/j.saa.2019.117637
Ayaz NO, Alnahdi HS (2011) RTICLES immobilization and properties of β-D-galactosidase from. 7:2448–2454
Barbano DM (2017) A 100-year review: the production of fluid (market) milk. J Dairy Sci 100:9894–9902. https://doi.org/10.3168/jds.2017-13561
Barbosa O, Ortiz C, Berenguer-Murcia Á, Torres R, Rodrigues RC, Fernandez-Lafuente R (2014) Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv 4:1583–1600. https://doi.org/10.1039/C3RA45991H
Basso A, Serban S (2019) Industrial applications of immobilized enzymes—a review. Mol Catal 479:110607. https://doi.org/10.1016/j.mcat.2019.110607
Cabral BV, Santos LD, Santana Falleiros LNS, Carmo TS, Freitas FF, Cardoso SL, Resende MM, Ribeiro EJ (2017) Sucrose hydrolysis by invertase immobilized on Duolite A-568 employing a packed-bed reactor. Chem Eng Commun 204:1007–1019. https://doi.org/10.1080/00986445.2017.1336089
Carević M, Ćorović M, Mihailović M, Banjanac K, Milisavljević A, Veličković D, Bezbradica D (2016) Galacto-oligosaccharide synthesis using chemically modified β-galactosidase from Aspergillus oryzae immobilised onto macroporous amino resin. Int Dairy J 54:50–57. https://doi.org/10.1016/j.idairyj.2015.10.002
Costa HCB, Romão BB, Ribeiro EJ, Resende MM (2013) Glutaraldehyde effect in the immobilization process of alpha-galactosidase from Aspergillus Niger in the ion exchange resin duolite A-568. Chem Eng Trans 32:1105–1110. https://doi.org/10.3303/CET1332185
de Albuquerque TL, Gomes SDL, D’Almeida AP, Fernandez-Lafuente R, Gonçalves LRB, Rocha MVP (2018) Immobilization of β-galactosidase in glutaraldehyde-chitosan and its application to the synthesis of lactulose using cheese whey as feedstock. Process Biochem 73:65–73. https://doi.org/10.1016/j.procbio.2018.08.010
De Albuquerque TL, Peirce S, Rueda N, Marzocchella A, Gonçalves LRB, Rocha MVP, Fernandez-Lafuente R (2016) Ion exchange of β-galactosidase: the effect of the immobilization pH on enzyme stability. Process Biochem 51:875–880. https://doi.org/10.1016/j.procbio.2016.03.014
De Sousa CC, Gonçalves GTI, Falleiros LNSS (2018) Ethanol production using agroindustrial residues as fermentation substrates by Kluyveromyces marxianus. Ind Biotechnol 14:308–314. https://doi.org/10.1089/ind.2018.0023
Elsayed EA, Danial EN, Wadaan MA, El-Enshasy HA (2019) Production of β-galactosidase in shake-flask and stirred tank bioreactor cultivations by a newly isolated Bacillus licheniformis strain. Biocatal Agric Biotechnol 20:101231. https://doi.org/10.1016/j.bcab.2019.101231
Eskandarloo H, Abbaspourrad A (2018) Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem 251:115–124. https://doi.org/10.1016/j.foodchem.2018.01.068
Falleiros LNSS, Cabral BV, Fischer J, Guidini CZ, Cardoso VL, De Resende MM, Ribeiro EJ (2017) Improvement of recovered activity and stability of the Aspergillus oryzae β-galactosidase immobilized on duolite® A568 by combination of immobilization methods. Chem Ind Chem Eng Q 23:495–506. https://doi.org/10.2298/CICEQ160912010F
Fischer J, Guidini CZ, Santana LNS, de Resende MM, Cardoso VL, Ribeiro EJ (2013) Optimization and modeling of lactose hydrolysis in a packed bed system using immobilized β-galactosidase from Aspergillus oryzae. J Mol Catal B Enzym 85–86:178–186. https://doi.org/10.1016/j.molcatb.2012.09.008
Gékas V, Lopez-Leiva M (1985) Hidrolysis of lactose: a literature review. Process Biochem 20:2–12
Gonçalves Filho D, Silva AG, Guidini CZ (2019) Lipases: sources, immobilization methods, and industrial applications. Appl Microbiol Biotechnol 103:7399–7423. https://doi.org/10.1007/s00253-019-10027-6
Guidini CZ, Fischer J, Santana LNS, Cardoso VL, Ribeiro EJ (2010) Immobilization of Aspergillus oryzae β-galactosidase in ion exchange resins by combined ionic-binding method and cross-linking. Biochem Eng J 52:137–143. https://doi.org/10.1016/j.bej.2010.07.013
Gürdaş S, Güleç HA, Mutlu M (2012) Immobilization of Aspergillus oryzae β-galactosidase onto Duolite A568 resin via simple adsorption mechanism. Food Bioprocess Technol 5:904–911. https://doi.org/10.1007/s11947-010-0384-7
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821. https://doi.org/10.1007/s10450-014-9623-y
Juajun O, Nguyen T-H, Maischberger T, Iqbal S, Haltrich D, Yamabhai M (2011) Cloning, purification, and characterization of β-galactosidase from Bacillus licheniformis DSM 13. Appl Microbiol Biotechnol 89:645–654. https://doi.org/10.1007/s00253-010-2862-2
Ladero M, Santos A, García-Ochoa F (2006) Kinetic modelling of the thermal inactivation of an industrial β-galactosidase from Kluyveromyces fragilis. Enzym Microb Technol 38:1–9. https://doi.org/10.1016/j.enzmictec.2004.03.031
Mahoney RR (1998) Galactosyl-oligosaccharide formation during lactose hydrolysis: a review. Food Chem 63:147–154
Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment☆. Enzym Microb Technol 26:509–515. https://doi.org/10.1016/S0141-0229(99)00188-X
Park C-S, Kwon H-J, Yeom S-J, Oh D-K (2010) Mannose production from fructose by free and immobilized d-lyxose isomerases from Providencia stuartii. Biotechnol Lett 32:1305–1309. https://doi.org/10.1007/s10529-010-0300-2
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: Why, what and how. Chem Soc Rev 42:6223–6235. https://doi.org/10.1039/c3cs60075k
Singh RS, Dhaliwal R, Puri M (2007) Production of high fructose syrup from Asparagus inulin using immobilized exoinulinase from Kluyveromyces marxianus YS-1. J Ind Microbiol Biotechnol 34:649–655. https://doi.org/10.1007/s10295-007-0237-1
Urrutia P, Bernal C, Wilson L, Illanes A (2018) Use of chitosan heterofunctionality for enzyme immobilization: β-galactosidase immobilization for galacto-oligosaccharide synthesis. Int J Biol Macromol 116:182–193. https://doi.org/10.1016/j.ijbiomac.2018.04.112
Wahba IM, Hassan EM (2015) Novel grafted agar disks for the covalent immobilization of β-D-galactosidase. Biopolymers 103:675–684. https://doi.org/10.1002/bip.22693
Acknowledgements
The authors wish to thank the CNPq and FAPEMIG, Brazil, for financial support.
Data availability statement (DAS)
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
LMK conducted the experimental work, and VPRR helped in preparing the experiments. EJR and M.G.S contributed with new reagents and methodology. RCS assisted in the treatment of results and statistical analysis. CZG, LNSSF, and LMK analyzed the data and wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuribayashi, L.M., do Rio Ribeiro, V.P., de Santana, R.C. et al. Immobilization of β-galactosidase from Bacillus licheniformis for application in the dairy industry. Appl Microbiol Biotechnol 105, 3601–3610 (2021). https://doi.org/10.1007/s00253-021-11325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11325-8