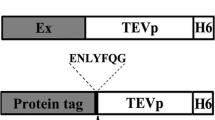
Abstract
The Escherichia coli (E. coli) expression system has been widely used to produce recombinant proteins. However, in some heterologous expressions, there are still difficulties in large-scale production. The use of fusion partners is one of the strategies for improving the expression levels of proteins in E. coli host. Here, we demonstrate a novel fusion element, the NT11-tag, which enhances protein expression. The NT11-tag was derived from the first 11 amino acid residues within the N-terminal N-half domain of a duplicated carbonic anhydrase (dCA) from Dunaliella species. Previously, we have found that the tag improves expression of the C-half domain of dCA when linked to its N-terminus. To verify its use as a protein production enhancer tag, two kinds of CAs derived from Hahella chejuensis (Hc-CA) and Thermovibrio ammonifican (Ta-CA) and the yellow fluorescent protein (YFP) were used as model proteins to measure their increased expression upon fusion with the NT11-tag. The NT11-tag amplified protein expression in E. coli by 6.9- and 7.6-fold for Ta-CA and YFP, respectively. Moreover, the tag also enhanced the soluble expression of Hc-CA, Ta-CA, and YFP by 1.7-, 5.0-, and 3.2-fold, respectively. Furthermore, protein yield was increased without inhibiting protein function. These results indicate that the use of the NT11-tag is a promising method for improving protein production in E. coli.





Similar content being viewed by others
References
Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J (2005) The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell 121(5):703–712. https://doi.org/10.1016/j.cell.2005.03.023
Bellaousov S, Reuter JS, Seetin MG, Mathews DH (2013) RNAstructure: web servers for RNA secondary structure prediction and analysis. Nucleic Acids Res 41(Web Server issue):W471–W474. https://doi.org/10.1093/nar/gkt290
Bivona L, Zou ZC, Stutzman N, Sun PD (2010) Influence of the second amino acid on recombinant protein expression. Protein Expr Purif 74(2):248–256. https://doi.org/10.1016/j.pep.2010.06.005
Butt TR, Edavettal SC, Hall JP, Mattern MR (2005) SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif 43(1):1–9. https://doi.org/10.1016/j.pep.2005.03.016
Cheng Y, Gu JA, Wang HG, Yu S, Liu YQ, Ning YL, Zou QM, Yu XJ, Mao XH (2010) EspA is a novel fusion partner for expression of foreign proteins in Escherichia coli. J Biotechnol 150(3):380–388. https://doi.org/10.1016/j.jbiotec.2010.09.940
Chou CP (2007) Engineering cell physiology to enhance recombinant protein production in Escherichia coli. Appl Microbiol Biotechnol 76(3):521–532. https://doi.org/10.1007/s00253-007-1039-0
Christendat D, Yee A, Dharamsi A, Kluger Y, Savchenko A, Cort JR, Booth V, Mackereth CD, Saridakis V, Ekiel I, Kozlov G, Maxwell KL, Wu N, McIntosh LP, Gehring K, Kennedy MA, Davidson AR, Pai EF, Gerstein M, Edwards AM, Arrowsmith CH (2000) Structural proteomics of an archaeon. Nat Struct Biol 7(10):903–909. https://doi.org/10.1038/82823
Costa SJ, Almeida A, Castro A, Domingues L, Besir H (2013) The novel Fh8 and H fusion partners for soluble protein expression in Escherichia coli: a comparison with the traditional gene fusion technology. Appl Microbiol Biotechnol 97(15):6779–6791. https://doi.org/10.1007/s00253-012-4559-1
Costa SJ, Almeida A, Castro A, Domingues L (2014) Fusion tags for protein solubility, purification and immunogenicity in Escherichia coli: the novel Fh8 system. Front Microbiol 5:63. https://doi.org/10.3389/fmicb.2014.00063
Demain AL, Vaishnav P (2009) Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv 27(3):297–306. https://doi.org/10.1016/j.biotechadv.2009.01.008
Di Fiore A, Alterio V, Monti SM, De Simone G, D'Ambrosio K (2015) Thermostable carbonic anhydrases in biotechnological applications. Int J Mol Sci 16(7):15456–15480. https://doi.org/10.3390/ijms160715456
Douette P, Navet R, Gerkens P, Galleni M, Levy D, Sluse FE (2005) Escherichia coli fusion carrier proteins act as solubilizing agents for recombinant uncoupling protein 1 through interactions with GroEL. Biochem Bioph Res Co 333(3):686–693. https://doi.org/10.1016/j.bbrc.2005.05.164
Dyson MR, Shadbolt SP, Vincent KJ, Perera RL, McCafferty J (2004) Production of soluble mammalian proteins in Escherichia coli: identification of protein features that correlate with successful expression. BMC Biotechnol 4:32. https://doi.org/10.1186/1472-6750-4-32
Esposito D, Chatterjee DK (2006) Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol 17(4):353–358. https://doi.org/10.1016/j.copbio.2006.06.003
Hu J, Qin H, Sharma M, Cross TA, Gao FP (2008) Chemical cleavage of fusion proteins for high-level production of transmembrane peptides and protein domains containing conserved methionines. Biochim Biophys Acta 1778(4):1060–1066. https://doi.org/10.1016/j.bbamem.2007.12.024
James P, Isupov MN, Sayer C, Saneei V, Berg S, Lioliou M, Kotlar HK, Littlechild JA (2014) The structure of a tetrameric alpha-carbonic anhydrase from Thermovibrio ammonificans reveals a core formed around intermolecular disulfides that contribute to its thermostability. Acta Crystallogr D Biol Crystallogr 70(Pt 10):2607–2618. https://doi.org/10.1107/S1399004714016526
Jo BH, Seo JH, Cha HJ (2014) Bacterial extremo-alpha-carbonic anhydrases from deep-sea hydrothermal vents as potential biocatalysts for CO2 sequestration. J Mol Catal B-Enzym 109:31–39. https://doi.org/10.1016/j.molcatb.2014.08.002
Jones MD, Fayerman JT (1987) Industrial applications of recombinant-DNA technology. J Chem Educ 64(4):337–339. https://doi.org/10.1021/ed064p337
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8(8):1668–1674. https://doi.org/10.1110/ps.8.8.1668
Kato A, Maki K, Ebina T, Kuwajima K, Soda K, Kuroda Y (2007) Mutational analysis of protein solubility enhancement using short peptide tags. Biopolymers 85(1):12–18. https://doi.org/10.1002/bip.20596
Ki MR, Min K, Kanth BK, Lee J, Pack SP (2013) Expression, reconstruction and characterization of codon-optimized carbonic anhydrase from Hahella chejuensis for CO2 sequestration application. Bioprocess Biosyst Eng 36(3):375–381. https://doi.org/10.1007/s00449-012-0788-z
Ki MR, Nguyen TKM, Kim SH, Kwon I, Pack SP (2016) Chimeric protein of internally duplicated alpha-type carbonic anhydrase from Dunaliella species for improved expression and CO2 sequestration. Process Biochem 51(9):1222–1229. https://doi.org/10.1016/j.procbio.2016.05.013
Kohl T, Schmidt C, Wiemann S, Poustka A, Korf U (2008) Automated production of recombinant human proteins as resource for proteome research. Proteome Sci 6:4. https://doi.org/10.1186/1477-5956-6-4
Kohyama K, Matsumoto T, Imoto T (2010) Refolding of an unstable lysozyme by gradient removal of a solubilizer and gradient addition of a stabilizer. J Biochem 147(3):426–431. https://doi.org/10.1093/jb/mvp184
Kozak M (2005) Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene 361:13–37. https://doi.org/10.1016/j.gene.2005.06.037
Kudla G, Murray AW, Tollervey D, Plotkin JB (2009) Coding-sequence determinants of gene expression in Escherichia coli. Science 324(5924):255–258. https://doi.org/10.1126/science.1170160
Kudou M, Yunmioka R, Ejima D, Arakawa T, Tsumoto K (2011) A novel protein refolding system using lauroyl-L-glutamate as a solubilizing detergent and arginine as a folding assisting agent. Protein Expr Purif 75(1):46–54. https://doi.org/10.1016/j.pep.2010.08.011
Li YF (2011) Self-cleaving fusion tags for recombinant protein production. Biotechnol Lett 33(5):869–881. https://doi.org/10.1007/s10529-011-0533-8
Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15(1):182–189. https://doi.org/10.1110/ps.051812706
Min KH, Son RG, Ki MR, Choi YS, Pack SP (2016) High expression and biosilica encapsulation of alkaline-active carbonic anhydrase for CO2 sequestration system development. Chemosphere 143:128–134. https://doi.org/10.1016/j.chemosphere.2015.07.020
Ohtake S, Kita Y, Arakawa T (2011) Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliv Rev 63(13):1053–1073. https://doi.org/10.1016/j.addr.2011.06.011
Peti W, Page R (2007) Strategies to maximize heterologous protein expression in Escherichia coli with minimal cost. Protein Expr Purif 51(1):1–10. https://doi.org/10.1016/j.pep.2006.06.024
Ramos R, Moreira S, Rodrigues A, Gama M, Domingues L (2013) Recombinant expression and purification of the antimicrobial peptide magainin-2. Biotechnol Prog 29(1):17–22. https://doi.org/10.1002/btpr.1650
Reddy RC, Lilie H, Rudolph R, Lange C (2005) L-arginine increases the solubility of unfolded species of hen egg white lysozyme. Protein Sci 14(4):929–935. https://doi.org/10.1110/ps.041085005
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5(172). https://doi.org/10.3389/fmicb.2014.00172
Seo SW, Yang JS, Kim I, Yang J, Min BE, Kim S, Jung GY (2013) Predictive design of mRNA translation initiation region to control prokaryotic translation efficiency. Metab Eng 15:67–74. https://doi.org/10.1016/j.ymben.2012.10.006
Sharma L, Sharma A (2001) Influence of cyclodextrin ring substituents on folding-related aggregation of bovine carbonic anhydrase. Eur J Biochem 268(8):2456–2463. https://doi.org/10.1046/j.1432-1327.2001.02125.x
Shine J, Dalgarno L (1975) Determinant of cistron specificity in bacterial ribosomes. Nature 254(5495):34–38. https://doi.org/10.1038/254034a0
Sorensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115(2):113–128. https://doi.org/10.1016/j.jbiotec.2004.08.004
Su Y, Zou ZR, Feng SY, Zhou P, Cao LJ (2007) The acidity of protein fusion partners predominantly determines the efficacy to improve the solubility of the target proteins expressed in Escherichia coli. J Biotechnol 129(3):373–382. https://doi.org/10.1016/j.jbiotec.2007.01.015
Terpe K (2003) Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60(5):523–533. https://doi.org/10.1007/s00253-002-1158-6
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72(2):211–222. https://doi.org/10.1007/s00253-006-0465-8
Tessema M, Simons PC, Cimino DF, Sanchez L, Waller A, Posner RG, Wandinger-Ness A, Prossnitz ER, Sklar LA (2006) Glutathione-S-transferase-green fluorescent protein fusion protein reveals slow dissociation from high site density beads and measures free GSH. Cytometry A 69(5):326–334. https://doi.org/10.1002/cyto.a.20259
Timasheff SN (2002) Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry 41(46):13473–13482. https://doi.org/10.1021/bi020316e
Tsumoto K, Ejima D, Kumagai I, Arakawa T (2003) Practical considerations in refolding proteins from inclusion bodies. Protein Expr Purif 28(1):1–8. https://doi.org/10.1016/S1046-5928(02)00641-1
Umetsu M, Tsumoto K, Hara M, Ashish K, Goda S, Adschiri T, Kumagai I (2003) How additives influence the refolding of immunoglobulin-folded proteins in a stepwise dialysis system—spectroscopic evidence for highly efficient refolding of a single-chain FV fragment. J Biol Chem 278(11):8979–8987. https://doi.org/10.1074/jbc.M212247200
Vandevenne M, Gaspard G, Belgsir el M, Ramnath M, Cenatiempo Y, Marechal D, Dumoulin M, Frere JM, Matagne A, Galleni M, Filee P (2011) Effects of monopropanediamino-beta-cyclodextrin on the denaturation process of the hybrid protein BlaPChBD. Biochim Biophys Acta 1814(9):1146–1153. https://doi.org/10.1016/j.bbapap.2011.05.007
Verpoorte JA (1967) Esterase activities of human carbonic anhydrases B and C. J Biol Chem 242(18):4221–4229
Waugh DS (2005) Making the most of affinity tags. Trends Biotechnol 23(6):316–320. https://doi.org/10.1016/j.tibtech.2005.03.012
Waugh DS (2011) An overview of enzymatic reagents for the removal of affinity tags. Protein Expr Purif 80(2):283–293. https://doi.org/10.1016/j.pep.2011.08.005
Wilbur KM, Anderson NG (1948) Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 176(1):147–154
Yamaguchi H, Miyazaki M, Briones-Nagata MP, Maeda H (2010) Refolding of difficult-to-fold proteins by a gradual decrease of denaturant using microfluidic chips. J Biochem 147(6):895–903. https://doi.org/10.1093/jb/mvq024
Yee A, Pardee K, Christendat D, Savchenko A, Edwards AM, Arrowsmith CH (2003) Structural proteomics: toward high-throughput structural biology as a tool in functional genomics. Acc Chem Res 36(3):183–189. https://doi.org/10.1021/ar010126g
Young CL, Britton ZT, Robinson AS (2012) Recombinant protein expression and purification: a comprehensive review of affinity tags and microbial applications. Biotechnol J 7(5):620–634. https://doi.org/10.1002/biot.201100155
Zhang YB, Howitt J, McCorkle S, Lawrence P, Springer K, Freimuth P (2004) Protein aggregation during overexpression limited by peptide extensions with large net negative charge. Protein Expr Purif 36(2):207–216. https://doi.org/10.1016/j.pep.2004.04.020
Funding
This work was supported by the Basic Core Technology Development Program for the Oceans and the Polar Regions of the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning, Korea (NRF-2015M1A5A1037054) and a Marine Biomaterials Research Center grant from the Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Korea. This work was also supported by Research Fellow Funding grant funded by the Ministry of Education, Korea (NRF-2014R1A1A2008088) and BK21 plus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 643 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T.K.M., Ki, M.R., Son, R.G. et al. The NT11, a novel fusion tag for enhancing protein expression in Escherichia coli. Appl Microbiol Biotechnol 103, 2205–2216 (2019). https://doi.org/10.1007/s00253-018-09595-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-09595-w