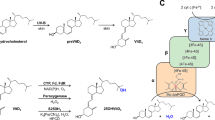
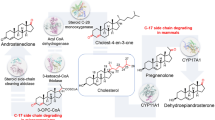
Abstract
Steroid C25 dehydrogenase (S25DH) from Sterolibacterium denitrificans Chol-1S is a molybdenum oxidoreductase belonging to the so-called ethylbenzene dehydrogenase (EBDH)-like subclass of DMSO reductases capable of the regioselective hydroxylation of cholesterol or cholecalciferol to 25-hydroxy products. Both products are important biologically active molecules: 25-hydroxycholesterol is responsible for a complex regulatory function in the immunological system, while 25-hydroxycholecalciferol (calcifediol) is the activated form of vitamin D3 used in the treatment of rickets and other calcium disorders. Studies revealed that the optimal enzymatic synthesis proceeds in fed-batch reactors under anaerobic conditions, with 6–9 % (w/v) 2-hydroxypropyl-β-cyclodextrin as a solubilizer and 1.25–5 % (v/v) 2-methoxyethanol as an organic co-solvent, both adjusted to the substrate type, and 8–15 mM K3[Fe(CN)6] as an electron acceptor. Such thorough optimization of the reaction conditions resulted in high product concentrations: 0.8 g/L for 25-hydroxycholesterol, 1.4 g/L for calcifediol and 2.2 g/L for 25-hydroxy-3-ketosterols. Although the purification protocol yields approximately 2.3 mg of pure S25DH from 30 g of wet cell mass (specific activity of 14 nmol min−1 mg−1), the non-purified crude extract or enzyme preparation can be readily used for the regioselective hydroxylation of both cholesterol and cholecalciferol. On the other hand, pure S25DH can be efficiently immobilized either on powder or a monolithic silica support functionalized with an organic linker providing NH2 groups for enzyme covalent binding. Although such immobilization reduced the enzyme initial activity more than twofold it extended S25DH catalytic lifetime under working conditions at least 3.5 times.







Similar content being viewed by others
References
Ban JG, Kim HB, Lee MJ, Anbu P, Kim ES (2014) Identification of a vitamin D3-specific hydroxylase genes through actinomycetes genome mining. J Ind Microbiol Biotechnol 41(2):265–273. doi:10.1007/s10295-013-1336-9
Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang GS, Russell DW (2009) 25-hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin a production. Proc Natl Acad Sci U S A 106(39):16764–16769. doi:10.1073/pnas.0909142106
Beste L, Nahar N, Dalman K, Fujioka S, Jonsson L, Dutta PC, Sitbon F (2011) Synthesis of hydroxylated sterols in transgenic Arabidopsis plants alters growth and steroid metabolism. Plant Physiol 157(1):426–440. doi:10.1104/pp.110.171199
Bildziukevich U, Rarova L, Saman D, Havlicek L, Drasar P, Wimmer Z (2013) Amides derived from heteroaromatic amines and selected steryl hemiesters. Steroids 78(14):1347–1352
Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Edel JO, Stähelin HB, Wolfram S, Jetter A, Schwager J, Henschkowski J, von Eckardstein A, Egli A (2012) Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res 27(1):160–169. doi:10.1002/jbmr.551
Brandi ML, Minisola S (2013) Calcidiol [25(OH)D3]: from diagnostic marker to therapeutical agent. Curr Med Res Opin 29(11):1565–1572. doi:10.1185/03007995.2013.838549
Brixius-Anderko S, Schiffer L, Hannemann F, Janocha B, Bernhardt R (2015) A CYP21A2 based whole-cell system in Escherichia coli for the biotechnological production of premedrol. Microb Cell Factories 14:135. doi:10.1186/s12934-015-0333-2
Campbell JA, Squires DM, Babcock JC (1969) Synthesis of 25-hydroxycholecalciferol biologically effective metabolite of vitamin D3. Steroids 13(5):567–577
Carvalho JF, Silva MM, Moreira JN, Simoes S, Sa e Melo ML (2010) Sterols as anticancer agents: synthesis of ring-B oxygenated steroids, cytotoxic profile, and comprehensive SAR analysis. J Med Chem 53(21):7632–7638. doi:10.1021/jm1007769
Chiang YR, Ismail W, Muller M, Fuchs G (2007) Initial steps in the anoxic metabolism of cholesterol by the denitrifying Sterolibacterium denitrificans. J Biol Chem 282(18):13240–13249. doi:10.1074/jbc.M610963200
Chiang YR, Ismail W, Gallien S, Heintz D, Van Dorsselaer A, Fuchs G (2008a) Cholest-4-en-3-one-Delta(1)-dehydrogenase, a flavoprotein catalyzing the second step in anoxic cholesterol metabolism. Appl Environ Microbiol 74(1):107–113
Chiang YR, Ismail W, Heintz D, Schaeffer C, Van Dorsselaer A, Fuchs G (2008b) Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J Bacteriol 190(3):905–914. doi:10.1128/jb.01525-07
Czarny MR, Nelson JA, Spencer TA (1977) Synthesis of 4-Spiro[Cyclopropanecholestan-3beta-Ol. J Org Chem 42(17):2941–2944. doi:10.1021/jo00437a041
Decaprio J, Yun J, Javitt NB (1992) Bile-acid and sterol solubilization in 2-hydroxypropyl-Beta-cyclodextrin. J Lipid Res 33(3):441–443
Dermer J, Fuchs G (2012) Molybdoenzyme that catalyzes the anaerobic hydroxylation of a tertiary carbon atom in the side chain of cholesterol. J Biol Chem 287(44):36905–36916
DiCosimo R, McAuliffe J, Poulose AJ, Bohlmann G (2013) Industrial use of immobilized enzymes. Chem Soc Rev 42(15):6437–6474. doi:10.1039/C3CS35506C
Donova MV (2007) Transformation of steroids by actinobacteria: a review. Appl Biochem Micro 43(1):1–14. doi:10.1134/S0003683807010012
Fujii T, Fujii Y, Machida K, Ochiai A, Ito M (2009) Efficient biotransformations using Escherichia coli with tolC acrAB mutations expressing cytochrome P450 genes. Biosci Biotechnol Biochem 73(4):805–810. doi:10.1271/bbb.80627
Heider J, Szaleniec M, Sünwoldt K, Boll M (2016) Ethylbenzene dehydrogenase and related molybdenum enzymes involved in oxygen-independent alkyl chain hydroxylation. J Mol Microbiol Biotechnol 26(1–3):45–62
Hille R, Hall J, Basu P (2014) The mononuclear molybdenum enzymes. Chem Rev 114(7):3963–4038. doi:10.1021/cr400443z
Holland HL (1992) Organic synthesis with oxidative enzymes. Wiley-VCH, New York
Iida T, Shinohara T, Goto J, Nambara T, Chang FC (1988) A facile one-step synthesis of delta-1,4-3-keto bile-acid esters by iodoxybenzene and benzeneselenic anhydride. J Lipid Res 29(8):1097–1101
Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoecklin E, Goessl R, Henschkowski J, Bischoff-Ferrari HA (2014) Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone 59:14–19
Kang D-J, Lee H-S, Park J-T, Bang JS, Hong S-K, Kim T-Y (2006) Optimization of culture conditions for the bioconversion of vitamin D3 to 1α,25-dihydroxyvitamin D3 using Pseudonocardia autotrophica ID 9302. Biotechnol Bioproc E 11(5):408–413. doi:10.1007/bf02932307
Kang DJ, Im JH, Kang JH, Kim KH (2015) Bioconversion of vitamin D3 to calcifediol by using resting cells of Pseudonocardia sp. Biotechnol Lett 37(9):1895–1904. doi:10.1007/s10529-015-1862-9
Kurek-Tyrlik A, Michalak K, Wicha J (2005) Synthesis of 17-epi-calcitriol from a common androstane derivative, involving the ring B photochemical opening and the intermediate triene ozonolysis. J Org Chem 70(21):8513–8521. doi:10.1021/jo051357u
McDonald JG, Russell DW (2010) Editorial: 25-hydroxycholesterol: a new life in immunology. J Leukoc Biol 88(6):1071–1072. doi:10.1189/jlb.0710418
Miyamoto K, Kubodera N, Murayama E, Ochi K, Mori T, Matsunaga I (1986) Synthetic studies on vitamin-D analogs .6. A new synthesis of 25-hydroxycholesterol from lithocholic acid. Synthetic Commun 16(5):513–521. doi:10.1080/00397918608078765
Neter J, Wasserman W, Kutner MH (1985) Applied linear statistical models: regression, analysis of variance, and experimental designs. Irwin, Homewood, IL
Ogawa S, Kakiyama G, Muto A, Hosoda A, Mitamura K, Ikegawa S, Hofmann AF, Iida T (2009) A facile synthesis of C-24 and C-25 oxysterols by in situ generated ethyl(trifluoroxymethyl)dioxirane. Steroids 74(1):81–87. doi:10.1016/j.steroids.2008.09.015
Rao SM, Thakkar K, Pawar K (2013) Microbial transformation of steroids: current trends in cortical side chain cleavage. Quest 1:16–20
Reboldi A, Dang EV, McDonald JG, Liang GS, Russell DW, Cyster JG (2014) 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345(6197):679–684. doi:10.1126/science.1254790
Riediker M, Schwartz J (1981) A new synthesis of 25-hydroxycholesterol. Tetrahedron Lett 22(46):4655–4658. doi:10.1016/S0040-4039(01)83005-7
Riva S (1991) Enzymatic modification of steroids., vol 1. Marcel Dekker, Inc., New York
Ryznar T, Krupa M, Kutner A (2002) Syntheses of vitamin D metabolites and analogs. Retrospect and prospects. Przem Chem 81(5):300–310
Sasaki J, Miyazaki A, Saito M, Adachi T, Mizoue K, Hanada K, Omura S (1992) Transformation of vitamin-D3 to 1-alpha, 25-dihydroxyvitamin-D3 via 25-hydroxyvitamin-D3 using Amycolata sp. strains. Appl Microbiol Biotechnol 38(2):152–157
Schilke KF, Kelly C (2008) Activation of immobilized lipase in non-aqueous systems by hydrophobic POIY-DL-tryptophan tethers. Biotechnol Bioeng 101(1):9–18. doi:10.1002/bit.21870
Seung-Kwon, N, Myung-Kuk, K, Won-Tae, Y, Kyung-Moon, P, Sang-Ok, P (2002) Method for preparation of androst-4-ene-3,17-dione and androsta-1,4-diene-3,17-dione. PCT/KR2002/000876
Szaleniec M, Hagel C, Menke M, Nowak P, Witko M, Heider J (2007) Kinetics and mechanism of oxygen-independent hydrocarbon hydroxylation by ethylbenzene dehydrogenase. Biochemistry 46(25):7637–7646. doi:10.1021/bi700633c
Szaleniec, M, Rugor, A, Dudzik, A, Tataruch, M, Szymańska, K, Jarzębski, A (2015) [Method of obtaining 25-hydroxylated sterol derivatives, including 25-hydroxy-7-dehydrocholesterol]. Poland
Szymanska K, Pudlo W, Mrowiec-Bialon J, Czardybon A, Kocurek J, Jarzebski AB (2013) Immobilization of invertase on silica monoliths with hierarchical pore structure to obtain continuous flow enzymatic microreactors of high performance. Micropor Mesopor Mat 170:75–82
Tarlera S (2003) Sterolibacterium denitrificans gen. Nov., sp. nov., a novel cholesterol-oxidizing, denitrifying member of the Proteobacteria. Int J Syst Evol Microbiol 53(4):1085–1091. doi:10.1099/ijs.0.02039-0
Tataruch M, Heider J, Bryjak J, Nowak P, Knack D, Czerniak A, Liesiene J, Szaleniec M (2014) Suitability of the hydrocarbon-hydroxylating molybdenum-enzyme ethylbenzene dehydrogenase for industrial chiral alcohol production. J Biotechnol 192:400–409. doi:10.1016/j.jbiotec.2014.06.021
Warnke M, Jung T, Dermer J, Hipp K, Jehmlich N, von Bergen M, Ferlaino S, Fries A, Müller M, Boll M (2016) 25-hydroxyvitamin D3 synthesis by enzymatic steroid side-chain hydroxylation with water. Angew Chem Int Ed Engl 55(5):1881–1884. doi:10.1002/ange.201510331
Westover EJ, Covey DF (2006) Synthesis of ent-25-hydroxycholesterol. Steroids 71(6):484–488. doi:10.1016/j.steroids.2006.01.007
Williams RO, Mahaguna V, Sriwongjanya M (1998) Characterization of an inclusion complex of cholesterol and hydroxypropyl-beta-cyclodextrin. Eur J Pharm Biopharm 46(3):355–360. doi:10.1016/S0939-6411(98)00033-2
Yamamoto S, Kurihara H, Mutoh T, Xing XH, Unno H (2005) Cholesterol recovery from inclusion complex of beta-cyclodextrin and cholesterol by aeration at elevated temperatures. Biochem Eng J 22(3):197–205
Yasuda K, Endo M, Ikushiro S, Kamakura M, Ohta M, Sakaki T (2013) UV-dependent production of 25-hydroxyvitamin D2 in the recombinant yeast cells expressing human CYP2R1. Biochem Biophys Res Commun 434(2):311–315. doi:10.1016/j.bbrc.2013.02.124
Yasutake Y, Nishioka T, Imoto N, Tamura T (2013) A single mutation at the ferredoxin binding site of P450 Vdh enables efficient biocatalytic production of 25-hydroxyvitamin D(3. Chembiochem 14(17):2284–2291. doi:10.1002/cbic.201300386
Zhang D, Zhang R, Zhang J, Chen L, Zhao C, Dong W, Zhao Q, Wu Q, Zhu D (2014) Engineering a hydroxysteroid dehydrogenase to improve its soluble expression for the asymmetric reduction of cortisone to 11β-hydrocortisone. Appl Microbiol Biotechnol 98(21):8879–8886. doi:10.1007/s00253-014-5967-1
Zhao Q, Ji L, Qian GP, Liu JG, Wang ZQ, WP Y, Chen XZ (2014) Investigation on the synthesis of 25-hydroxycholesterol. Steroids 85:1–5. doi:10.1016/j.steroids.2014.02.002
Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF (2013) CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A 110(39):15650–15655. doi:10.1073/pnas.1315006110
Acknowledgments
The authors acknowledge the financial support of the Polish institutions of the National Center of Research and Development (grant project: LIDER/33/147/L-3/11/NCBR) and the National Center of Science (grant SONATA UMO-2012/05/D/ST4/00277).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interests.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic Supplementary Materials
ESM 1
(DOCX 1.85 mb)
Rights and permissions
About this article
Cite this article
Rugor, A., Tataruch, M., Staroń, J. et al. Regioselective hydroxylation of cholecalciferol, cholesterol and other sterol derivatives by steroid C25 dehydrogenase. Appl Microbiol Biotechnol 101, 1163–1174 (2017). https://doi.org/10.1007/s00253-016-7880-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7880-2