Abstract
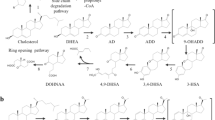
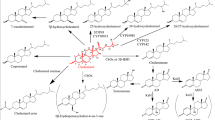
Cholesterol, an essential lipid for mammalian cells, can be used as a carbon source by bacteria and as a precursor for steroid hormones in animal cells. The cholesterol C17 side-chain-cleavage pathways are the committed and rate-limiting steps in the biosynthesis of steroid drugs. Three cholesterol C17 side-chain degradation pathways have been identified in nature: the β-oxidation pathways in actinobacteria, the oxygen-independent degradation pathway in Sterolibacterium denitrificans, and the cholesterol side-chain degradation pathway in mammals. An in-depth understanding of the structures and molecular mechanisms of enzymes in these degradation processes will facilitate the creation of enzyme mutants with better catalytic capabilities. The introduction of the engineered enzymes into the microbial cell factories may contribute to the industrial production of steroid drugs.
Graphical abstract











Similar content being viewed by others
References
Szaleniec M, Wojtkiewicz AM, Bernhardt R, Borowski T, Donova M. Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms. Appl Microbiol Biotechnol. 2018;102(19):8153–71. https://doi.org/10.1007/s00253-018-9239-3.
Vil VA, Gloriozova TA, Poroikov VV, Terent’ev AO, Savidov N, Dembitsky VM. Peroxy steroids derived from plant and fungi and their biological activities. Appl Microbiol Biotechnol. 2018;102(18):7657–67. https://doi.org/10.1007/s00253-018-9211-2.
Wang Y, McAllister TA, Yanke LJ, Cheeke PR. Effect of steroidal saponin from Yucca schidigera extract on ruminal microbes. J Appl Microbiol. 2000;88(5):887–96. https://doi.org/10.1046/j.1365-2672.2000.01054.x.
Gwynne JT, Strauss J. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3(3):299–329. https://doi.org/10.1210/edrv-3-3-299.
Bhatti HN, Khera RA. Biological transformations of steroidal compounds: a review. Steroids. 2012;77(12):1267–90. https://doi.org/10.1016/j.steroids.2012.07.018.
Mensah-Nyagan AG, Meyer L, Schaeffer V, Kibaly C, Patte-Mensah C. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinology. 2009;34(Suppl 1):S169–77. https://doi.org/10.1016/j.psyneuen.2009.06.004.
Jeal W, Faulds D. Triamcinolone acetonide. A review of its pharmacological properties and therapeutic efficacy in the management of allergic rhinitis. Drugs. 1997;53(2):257–80. https://doi.org/10.2165/00003495-199753020-00006.
Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D. Immune effects of corticosteroids in sepsis. Front Immunol. 2018;9:1736. https://doi.org/10.3389/fimmu.2018.01736.
Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis, and development. Annu Rev Biochem. 2011;80:885–916. https://doi.org/10.1146/annurev-biochem-081308-165917.
García JL, Uhía I, Galán B. Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb Biotechnol. 2012;5(6):679–99. https://doi.org/10.1111/j.1751-7915.2012.00331.x.
Shtratnikova VY, Bragin EY, Dovbnya DV, Pekov YA, Schelkunov MI, Strizhov N, Ivashina TV, Ashapkin VV, Donova MV. Complete genome sequence of sterol-transforming Mycobacterium neoaurum strain VKM Ac-1815D. Genome Announc. 2014;2(1):e01177-e1213. https://doi.org/10.1128/genomeA.01177-13.
Macías FA, Chinchilla N, Varela RM, Molinillo JM. Bioactive steroids from Oryza sativa L. Steroids. 2006;71(7):603–8. https://doi.org/10.1016/j.steroids.2006.03.001.
el El-Desoky SI, Reyad M, Afsah EM, Dawidar AA. Synthesis and chemical reactions of the steroidal hormone 17α-methyltestosterone. Steroids. 2016;105:68–95. https://doi.org/10.1016/j.steroids.2015.11.004.
Holert J, Brown K, Hashimi A, Eltis LD, Mohn WW. Steryl ester formation and accumulation in steroid-degrading bacteria. Appl Environ Microbiol. 2020;86(2):e02353-e2419. https://doi.org/10.1128/aem.02353-19.
Zhao A, Zhang X, Li Y, Wang Z, Lv Y, Liu J, Alam MA, Xiong W, Xu J. Mycolicibacterium cell factory for the production of steroid-based drug intermediates. Biotechnol Adv. 2021;53: 107860. https://doi.org/10.1016/j.biotechadv.2021.107860.
Warnke M, Jacoby C, Jung T, Agne M, Mergelsberg M, Starke R, Jehmlich N, von Bergen M, Richnow HH, Brüls T, Boll M. A patchwork pathway for oxygenase-independent degradation of side chain containing steroids. Environ Microbiol. 2017;19(11):4684–99. https://doi.org/10.1111/1462-2920.13933.
Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. https://doi.org/10.1210/er.2010-0013.
Batth R, Nicolle C, Cuciurean IS, Simonsen HT. Biosynthesis and industrial production of androsteroids. Plants (Basel). 2020;9(9):1144. https://doi.org/10.3390/plants9091144.
Sih CJ, Tai HH, Tsong YY, Lee SS, Coombe RG. Mechanisms of steroid oxidation by microorganisms. XIV. Pathway of cholesterol side-chain degradation. Biochemistry. 1968;7(2):808–18. https://doi.org/10.1021/bi00842a039.
Rosłoniec KZ, Wilbrink MH, Capyk JK, Mohn WW, Ostendorf M, van der Geize R, Dijkhuizen L, Eltis LD. Cytochrome P450 125 (CYP125) catalyses C26-hydroxylation to initiate sterol side-chain degradation in Rhodococcus jostii RHA1. Mol Microbiol. 2009;74(5):1031–43. https://doi.org/10.1111/j.1365-2958.2009.06915.x.
Chen YR, Huang HH, Cheng YF, Tang TY, Liu WH. Expression of a cholesterol oxidase gene from Arthrobacter simplex in Escherichia coli and Pichia pastoris. Enzyme Microb Technol. 2006;39(4):854–60. https://doi.org/10.1016/j.enzmictec.2006.01.018.
Sharma P, Slathia PS, Somal P, Mehta P. Biotransformation of cholesterol to 1,4-androstadiene-3,17-dione (ADD) by Nocardia species. Ann Microbiol. 2012;62(4):1651–9. https://doi.org/10.1007/s13213-012-0422-y.
Thomas ST, Yang X, Sampson NS. Inhibition of the M. tuberculosis 3β-hydroxysteroid dehydrogenase by azasteroids. Bioorg Med Chem Lett. 2011;21(8):2216–9. https://doi.org/10.1016/j.bmcl.2011.03.004.
Doukyu N. Characteristics and biotechnological applications of microbial cholesterol oxidases. Appl Microbiol Biotechnol. 2009;83(5):825–37. https://doi.org/10.1007/s00253-009-2059-8.
Chang J, Miner M, Pandey A, Gill W, Harik N, Sassetti C, Sherman D. igr genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol. 2009;191(16):5232–9. https://doi.org/10.1128/JB.00452-09.
Capyk JK, Kalscheuer R, Stewart GR, Liu J, Kwon H, Zhao R, Okamoto S, Jacobs WR Jr, Eltis LD, Mohn WW. Mycobacterial cytochrome p450 125 (cyp125) catalyzes the terminal hydroxylation of c27 steroids. J Biol Chem. 2009;284(51):35534–42. https://doi.org/10.1074/jbc.M109.072132.
Driscoll MD, McLean KJ, Levy C, Mast N, Pikuleva IA, Lafite P, Rigby SE, Leys D, Munro AW. Structural and biochemical characterization of Mycobacterium tuberculosis CYP142: evidence for multiple cholesterol 27-hydroxylase activities in a human pathogen. J Biol Chem. 2010;285(49):38270–82. https://doi.org/10.1074/jbc.M110.164293.
Johnston JB, Kells PM, Podust LM, Ortiz de Montellano PR. Biochemical and structural characterization of CYP124: a methyl-branched lipid omega-hydroxylase from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2009;106(49):20687–92. https://doi.org/10.1073/pnas.0907398106.
Casabon I, Swain K, Crowe AM, Eltis LD, Mohn WW. Actinobacterial acyl coenzyme A synthetases involved in steroid side-chain catabolism. J Bacteriol. 2014;196(3):579–87. https://doi.org/10.1128/jb.01012-13.
Johnston JB, Ouellet H, Ortiz de Montellano PR. Functional redundancy of steroid C26-monooxygenase activity in Mycobacterium tuberculosis revealed by biochemical and genetic analyses. J Biol Chem. 2010;285(47):36352–60. https://doi.org/10.1074/jbc.M110.161117.
Wrońska N, Brzostek A, Szewczyk R, Soboń A, Dziadek J, Lisowska K. The role of fadD19 and echA19 in sterol side chain degradation by Mycobacterium smegmatis. Molecules. 2016;21(5):598. https://doi.org/10.3390/molecules21050598.
Yang M, Lu R, Guja KE, Wipperman MF, St Clair JR, Bonds AC, Garcia-Diaz M, Sampson NS. Unraveling cholesterol catabolism in Mycobacterium tuberculosis: ChsE4-ChsE5 α2β2 acyl-CoA dehydrogenase initiates β-oxidation of 3-Oxo-cholest-4-en-26-oyl CoA. ACS Infect Dis. 2015;1(2):110–25. https://doi.org/10.1021/id500033m.
Kendall SL, Withers M, Soffair CN, Moreland NJ, Gurcha S, Sidders B, Frita R, Ten Bokum A, Besra GS, Lott JS, Stoker NG. A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis. Mol Microbiol. 2007;65(3):684–99. https://doi.org/10.1111/j.1365-2958.2007.05827.x.
Yuan T, Yang M, Gehring K, Sampson NS. Mycobacterium tuberculosis exploits a heterohexameric enoyl-CoA hydratase retro-aldolase complex for cholesterol catabolism. Biochemistry. 2019;58(41):4224–35. https://doi.org/10.1021/acs.biochem.9b00673.
Bonds AC, Yuan T, Werman JM, Jang J, Lu R, Nesbitt NM, Garcia-Diaz M, Sampson NS. Post-translational succinylation of Mycobacterium tuberculosis enoyl-CoA hydratase EchA19 slows catalytic hydration of cholesterol catabolite 3-Oxo-chol-4,22-diene-24-oyl-CoA. ACS Infect Dis. 2020;6(8):2214–24. https://doi.org/10.1021/acsinfecdis.0c00329.
Yuan T, Werman JM, Yin X, Yang M, Garcia-Diaz M, Sampson NS. Enzymatic β-oxidation of the cholesterol side chain in Mycobacterium tuberculosis bifurcates stereospecifically at hydration of 3-Oxo-cholest-4,22-dien-24-oyl-CoA. ACS Infect Dis. 2021;7(6):1739–51. https://doi.org/10.1021/acsinfecdis.1c00069.
Yang M, Guja KE, Thomas ST, Garcia-Diaz M, Sampson NS. A distinct MaoC-like enoyl-CoA hydratase architecture mediates cholesterol catabolism in Mycobacterium tuberculosis. ACS Chem Biol. 2014;9(11):2632–45. https://doi.org/10.1021/cb500232h.
Xu LQ, Liu YJ, Yao K, Liu HH, Tao XY, Wang FQ, Wei DZ. Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Sci Rep. 2016;6:21928. https://doi.org/10.1038/srep21928.
Anbazhagan P, Harijan RK, Kiema TR, Janardan N, Murthy MR, Michels PA, Juffer AH, Wierenga RK. Phylogenetic relationships and classification of thiolases and thiolase-like proteins of Mycobacterium tuberculosis and Mycobacterium smegmatis. Tuberculosis (Edinb). 2014;94(4):405–12. https://doi.org/10.1016/j.tube.2014.03.003.
Schaefer CM, Lu R, Nesbitt NM, Schiebel J, Sampson NS, Kisker C. FadA5 a thiolase from Mycobacterium tuberculosis: a steroid-binding pocket reveals the potential for drug development against tuberculosis. Structure. 2015;23(1):21–33. https://doi.org/10.1016/j.str.2014.10.010.
Aggett R, Mallette E, Gilbert SE, Vachon MA, Schroeter KL, Kimber MS, Seah SYK. The steroid side-chain-cleaving aldolase Ltp2–ChsH2(DUF35) is a thiolase superfamily member with a radically repurposed active site. J Biol Chem. 2019;294(31):11934–43. https://doi.org/10.1074/jbc.RA119.008889.
Gilbert S, Hood L, Seah SYK. Characterization of an aldolase involved in cholesterol side chain degradation in Mycobacterium tuberculosis. J Bacteriol. 2018;200(2):e00512-e517. https://doi.org/10.1128/jb.00512-17.
Ruprecht A, Maddox J, Stirling AJ, Visaggio N, Seah SY. Characterization of novel acyl coenzyme A dehydrogenases involved in bacterial steroid degradation. J Bacteriol. 2015;197(8):1360–7. https://doi.org/10.1128/jb.02420-14.
Thomas ST, Sampson NS. Mycobacterium tuberculosis utilizes a unique heterotetrameric structure for dehydrogenation of the cholesterol side chain. Biochemistry. 2013;52(17):2895–904. https://doi.org/10.1021/bi4002979.
Lu R, Schmitz W, Sampson NS. α-Methyl acyl CoA racemase provides Mycobacterium tuberculosis catabolic access to cholesterol esters. Biochemistry. 2015;54(37):5669–72. https://doi.org/10.1021/acs.biochem.5b00911.
Chiang YR, Ismail W, Gallien S, Heintz D, Van Dorsselaer A, Fuchs G. Cholest-4-en-3-one-delta 1-dehydrogenase, a flavoprotein catalyzing the second step in anoxic cholesterol metabolism. Appl Environ Microbiol. 2008;74(1):107–13. https://doi.org/10.1128/aem.01968-07.
Chiang YR, Ismail W, Müller M, Fuchs G. Initial steps in the anoxic metabolism of cholesterol by the denitrifying Sterolibacterium denitrificans. J Biol Chem. 2007;282(18):13240–9. https://doi.org/10.1074/jbc.M610963200.
Dermer J, Fuchs G. Molybdoenzyme that catalyzes the anaerobic hydroxylation of a tertiary carbon atom in the side chain of cholesterol. J Biol Chem. 2012;287(44):36905–16. https://doi.org/10.1074/jbc.M112.407304.
Lin CW, Wang PH, Ismail W, Tsai YW, El Nayal A, Yang CY, Yang FC, Wang CH, Chiang YR. Substrate uptake and subcellular compartmentation of anoxic cholesterol catabolism in Sterolibacterium denitrificans. J Biol Chem. 2015;290(2):1155–69. https://doi.org/10.1074/jbc.M114.603779.
Mast N, Annalora AJ, Lodowski DT, Palczewski K, Stout CD, Pikuleva IA. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J Biol Chem. 2011;286(7):5607–13. https://doi.org/10.1074/jbc.M110.188433.
Lee-Robichaud P, Shyadehi AZ, Wright JN, Akhtar ME, Akhtar M. Mechanistic kinship between hydroxylation and desaturation reactions: acyl-carbon bond cleavage promoted by pig and human CYP17 (P-45017α; 17α-hydroxylase-17,20-lyase). Biochemistry. 1995;34(43):14104–13. https://doi.org/10.1021/bi00043a015.
Swart P, Swart AC, Waterman MR, Estabrook RW, Mason JI. Progesterone 16α-hydroxylase activity is catalyzed by human cytochrome P450 17α-hydroxylase. J Clin Endocrinol Metab. 1993;77(1):98–102. https://doi.org/10.1210/jcem.77.1.8325965.
Ouellet H, Guan S, Johnston JB, Chow ED, Kells PM, Burlingame AL, Cox JS, Podust LM, de Montellano PR. Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest-4-en-3-one. Mol Microbiol. 2010;77(3):730–42. https://doi.org/10.1111/j.1365-2958.2010.07243.x.
García-Fernández E, Frank DJ, Galán B, Kells PM, Podust LM, García JL, de Montellano PR. A highly conserved mycobacterial cholesterol catabolic pathway. Environ Microbiol. 2013;15(8):2342–59. https://doi.org/10.1111/1462-2920.12108.
Varaksa T, Bukhdruker S, Grabovec I, Marin E, Kavaleuski A, Gusach A, Kovalev K, Maslov I, Luginina A, Zabelskii D, Astashkin R, Shevtsov M, Smolskaya S, Kavaleuskaya A, Shabunya P, Baranovsky A, Dolgopalets V, Charnou Y, Savachka A, Litvinovskaya R, Hurski A, Shevchenko E, Rogachev A, Mishin A, Gordeliy V, Gabrielian A, Hurt DE, Nikonenko B, Majorov K, Apt A, Rosenthal A, Gilep A, Borshchevskiy V, Strushkevich N. Metabolic fate of human immunoactive sterols in Mycobacterium tuberculosis. J Mol Biol. 2021;433(4): 166763. https://doi.org/10.1016/j.jmb.2020.166763.
Lu R, Schaefer CM, Nesbitt NM, Kuper J, Kisker C, Sampson NS. Catabolism of the cholesterol side chain in Mycobacterium tuberculosis is controlled by a redox-sensitive thiol switch. ACS Infect Dis. 2017;3(9):666–75. https://doi.org/10.1021/acsinfecdis.7b00072.
Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci USA. 2011;108(25):10139–43. https://doi.org/10.1073/pnas.1019441108.
Petrunak EM, DeVore NM, Porubsky PR, Scott EE. Structures of human steroidogenic cytochrome P450 17A1 with substrates. J Biol Chem. 2014;289(47):32952–64. https://doi.org/10.1074/jbc.M114.610998.
McLean KJ, Lafite P, Levy C, Cheesman MR, Mast N, Pikuleva IA, Leys D, Munro AW. The Structure of Mycobacterium tuberculosis CYP125: molecular basis for cholesterol binding in a P450 needed for host infection. J Biol Chem. 2009;284(51):35524–33. https://doi.org/10.1074/jbc.M109.032706.
Rittle J, Green MT. Cytochrome P450 compound I: capture, characterization, and C–H bond activation kinetics. Science. 2010;330(6006):933–7. https://doi.org/10.1126/science.1193478.
Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev. 2004;104(9):3947–80. https://doi.org/10.1021/cr020443g.
Sushko T, Kavaleuski A, Grabovec I, Kavaleuskaya A, Vakhrameev D, Bukhdruker S, Marin E, Kuzikov A, Masamrekh R, Shumyantseva V, Tsumoto K, Borshchevskiy V, Gilep A, Strushkevich N. A new twist of rubredoxin function in M. tuberculosis. Bioorg Chem. 2021;109:104721. https://doi.org/10.1016/j.bioorg.2021.104721.
Kim JJ, Miura R. Acyl-CoA dehydrogenases and acyl-CoA oxidases. Structural basis for mechanistic similarities and differences. Eur J Biochem. 2004;271(3):483–93. https://doi.org/10.1046/j.1432-1033.2003.03948.x.
Kim JJ, Wang M, Paschke R. Crystal structures of medium-chain acyl-CoA dehydrogenase from pig liver mitochondria with and without substrate. Proc Natl Acad Sci USA. 1993;90(16):7523–7. https://doi.org/10.1073/pnas.90.16.7523.
Headlam MJ, Wilce MC, Tuckey RC. The F–G loop region of cytochrome P450scc (CYP11A1) interacts with the phospholipid membrane. Biochim Biophys Acta. 2003;1617(1–2):96–108. https://doi.org/10.1016/j.bbamem.2003.09.007.
Usanov SA, Graham SE, Lepesheva GI, Azeva TN, Strushkevich NV, Gilep AA, Estabrook RW, Peterson JA. Probing the interaction of bovine cytochrome P450scc (CYP11A1) with adrenodoxin: evaluating site-directed mutations by molecular modeling. Biochemistry. 2002;41(26):8310–20. https://doi.org/10.1021/bi0255928.
Yoshimoto FK, Jung IJ, Goyal S, Gonzalez E, Guengerich FP. Isotope-labeling studies support the electrophilic compound I iron active species (FeO3+) for the carbon-carbon bond cleavage reaction of the cholesterol side-chain cleavage enzyme, cytochrome P450 11A1. J Am Chem Soc. 2016;138(37):12124–41. https://doi.org/10.1021/jacs.6b04437.
Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of P450c17 from four primate species. Endocrinology. 2002;143(12):4665–72. https://doi.org/10.1210/en.2002-220456.
Akhtar M, Wright JN, Lee-Robichaud P. A review of mechanistic studies on aromatase (CYP19) and 17α-hydroxylase-17,20-lyase (CYP17). J Steroid Biochem Mol Biol. 2011;125(1–2):2–12. https://doi.org/10.1016/j.jsbmb.2010.11.003.
DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482(7383):116–9. https://doi.org/10.1038/nature10743.
Meunie B. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem Rev. 2004;104(9):3947–80. https://doi.org/10.1021/cr020443g.
Estrada DF, Skinner AL, Laurence JS, Scott EE. Human cytochrome P450 17A1 conformational selection: modulation by ligand and cytochrome b5. J Biol Chem. 2014;289(20):14310–20. https://doi.org/10.1074/jbc.M114.560144.
Duggal R, Liu Y, Gregory MC, Denisov IG, Kincaid JR, Sligar SG. Evidence that cytochrome b5 acts as a redox donor in CYP17A1 mediated androgen synthesis. Biochem Biophys Res Commun. 2016;477(2):202–8. https://doi.org/10.1016/j.bbrc.2016.06.043.
Yao K, Xu LQ, Wang FQ, Wei DZ. Characterization and engineering of 3-ketosteroid-Δ1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3,17-dione through the catabolism of sterols. Metab Eng. 2014;24:181–91. https://doi.org/10.1016/j.ymben.2014.05.005.
Andor A, Jekkel A, Hopwood DA, Jeanplong F, Ilkoy E, Kónya A, Kurucz I, Ambrus G. Generation of useful insertionally blocked sterol degradation pathway mutants of fast-growing mycobacteria and cloning, characterization, and expression of the terminal oxygenase of the 3-ketosteroid 9α-hydroxylase in Mycobacterium smegmatis mc2155. Appl Environ Microbiol. 2006;72(10):6554–9. https://doi.org/10.1128/aem.00941-06.
Galán B, Uhía I, García-Fernández E, Martínez I, Bahíllo E, de la Fuente JL, Barredo JL, Fernández-Cabezón L, García JL. Mycobacterium smegmatis is a suitable cell factory for the production of steroidic synthons. Microb Biotechnol. 2017;10(1):138–50. https://doi.org/10.1111/1751-7915.12429.
Yao K, Wang FQ, Zhang HC, Wei DZ. Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng. 2013;15:75–87. https://doi.org/10.1016/j.ymben.2012.10.005.
Su L, Shen Y, Xia M, Shang Z, Xu S, An X, Wang M. Overexpression of cytochrome P450 125 in Mycobacterium: a rational strategy in the promotion of phytosterol biotransformation. J Ind Microbiol Biotechnol. 2018;45(10):857–67. https://doi.org/10.1007/s10295-018-2063-z.
Liu M, Xiong LB, Tao X, Liu QH, Wang FQ, Wei DZ. Integrated transcriptome and proteome studies reveal the underlying mechanisms for sterol catabolism and steroid production in Mycobacterium neoaurum. J Agric Food Chem. 2018;66(34):9147–57. https://doi.org/10.1021/acs.jafc.8b02714.
Szczebara FM, Chandelier C, Villeret C, Masurel A, Bourot S, Duport C, Blanchard S, Groisillier A, Testet E, Costaglioli P, Cauet G, Degryse E, Balbuena D, Winter J, Achstetter T, Spagnoli R, Pompon D, Dumas B. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol. 2003;21(2):143–9. https://doi.org/10.1038/nbt775.
Zhang R, Zhang Y, Wang Y, Yao M, Zhang J, Liu H, Zhou X, Xiao W, Yuan Y. Pregnenolone overproduction in Yarrowia lipolytica by integrative components pairing of the cytochrome P450scc system. ACS Synth Biol. 2019;8(12):2666–78. https://doi.org/10.1021/acssynbio.9b00018.
Yang J, Li C, Zhang Y. Engineering of Saccharomyces cerevisiae for 24-methylene-cholesterol production. Biomolecules. 2021;11(11):1710. https://doi.org/10.3390/biom11111710.
Su W, Xiao WH, Wang Y, Liu D, Zhou X, Yuan YJ. Alleviating redox imbalance enhances 7-dehydrocholesterol production in engineered Saccharomyces cerevisiae. PLoS One. 2015;10(6): e0130840. https://doi.org/10.1371/journal.pone.0130840.
Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat Rev Genet. 2010;11(5):367–79. https://doi.org/10.1038/nrg2775.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308. https://doi.org/10.1038/nprot.2013.143.
Gavira JA. Current trends in protein crystallization. Arch Biochem Biophys. 2016;602:3–11. https://doi.org/10.1016/j.abb.2015.12.010.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9. https://doi.org/10.1038/s41586-021-03819-2.
Farwell CC, McIntosh JA, Hyster TK, Wang ZJ, Arnold FH. Enantioselective imidation of sulfides via enzyme-catalyzed intermolecular nitrogen-atom transfer. J Am Chem Soc. 2014;136(24):8766–71. https://doi.org/10.1021/ja503593n.
Reetz MT. Controlling the enantioselectivity of enzymes by directed evolution: practical and theoretical ramifications. Proc Natl Acad Sci USA. 2004;101(16):5716–22. https://doi.org/10.1073/pnas.0306866101.
Funding
This work was financially supported by the National Key Research and Development Program of China (2019YFA0905300) and the Natural Science Foundation of China (no. 81872779).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kong, K., Zhang, M., Zhang, H. et al. Structures and molecular mechanisms of action of the cholesterol C17 side-chain-degrading enzymes. Syst Microbiol and Biomanuf 4, 1–19 (2024). https://doi.org/10.1007/s43393-022-00083-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00083-x