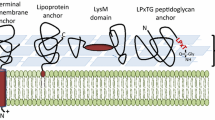
Abstract
Microbial cell surface display has attracted greater attention than ever and has numerous potential applications in biotechnology. With the safety and probiotic properties, lactic acid bacteria (LAB) have been used widely in food and industrial applications. In order to circumvent using genetically modified microorganisms which face low public acceptance and severe regulatory scrutiny, surface-engineered LAB without genetical modification are more preferred. According to the way used to obtain the fusion protein containing the passenger molecule and anchoring domain, the genetic or chemical approaches can be used to construct these surface-engineered LAB. In addition to the viable wide-type LAB, non-living bacterial-like particles (BLP) can be attached by these fusion proteins added from outside. Compared to the living LAB, BLP have a higher binding capacity and less anticarrier response. Mucosal vaccines are the predominant application of these surface-engineered LAB with no genetical modification.



Similar content being viewed by others
References
Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, Tomio K, Kojima S, Oda K, Sewaki T, Yasugi T, Kozuma S, Taketani Y (2010) Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 28(16):2810–2817. doi:10.1016/j.vaccine.2010.02.005
Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, Fuller S, Cebelinski EA, Chen Q, Jorba J, Kew OM, Pallansch MA, Oberste MS, Schleiss M, Davis JP, Warshawsky B, Squires S, Hull HF, Invest V-DP (2009) Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 199(3):391–397. doi:10.1086/596052
Antikainen J, Anton L, Sillanpaa J, Korhonen TK (2002) Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol Microbiol 46(2):381–394
Ashrafi F, Fallah Mehrabadi J, Siadat SD, Aghasadeghi MR (2015) Expression and purification of the uropathogenic Escherichia coli PapG protein and its surface absorption on Lactobacillus reuteri: implications for surface display system vaccines. Jundishapur J Microbiol 8(9):e25595. doi:10.5812/jjm.25595
Audouy SAL, van Roosmalen ML, Neef J, Kanninga R, Post E, Deemter M, Metselaar H, van Selm S, Robillard GT, Leenhouts KJ, Hermans PWM (2006) Lactococcus lactis GEM particles displaying pneumococcal antigens induce local and systemic immune responses following intranasal immunization. Vaccine 24(26):5434–5441. doi:10.1016/j.vaccine.2006.03.054
Audouy SAL, van Selm S, van Roosmalen ML, Post E, Kanninga R, Neef J, Estevao S, Nieuwenhuis EES, Adrian PV, Leenhouts K, Hermans PWM (2007) Development of lactococcal GEM-based pneumococcal vaccines. Vaccine 25(13):2497–2506. doi:10.1016/j.vaccine.2006.09.026
Avall-Jaaskelainen S, Palva A (2005) Lactobacillus surface layers and their applications. FEMS Microbiol Rev 29(3):511–529. doi:10.1016/j.femsre.2005.04.003
Avall-Jaaskelainen S, Kyla-Nikkila K, Kahala M, Miikkulainen-Lahti T, Palva A (2002) Surface display of foreign epitopes on the Lactobacillus brevis S-layer. Appl Environ Microbiol 68(12):5943–5951. doi:10.1128/Aem.68.12.5943-5951.2002
Bahey-El-Din M (2012) Lactococcus lactis-based vaccines from laboratory bench to human use: an overview. Vaccine 30(4):685–690. doi:10.1016/j.vaccine.2011.11.098
Bateman A, Bycroft M (2000) The structure of a LysM domain from E-coli membrane-bound lytic murein transglycosylase D (MltD. J Mol Biol 299(4):1113–1119. doi:10.1006/jmbi.2000.3778
Berlec A, Zadravec P, Jevnikar Z, Strukelj B (2011) Identification of candidate carrier proteins for surface display on Lactococcus lactis by theoretical and experimental analyses of the surface proteome. Appl Environ Microbiol 77(4):1292–1300. doi:10.1128/AEM.02102-10
Bermudez-Humaran LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, Chatel JM, Langella P (2013) Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol 16(3):278–283. doi:10.1016/j.mib.2013.06.002
Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, Alcocer-Gonzalez JM, Tamez-Guerra RS, de Oca-Luna RM, Langella P (2004) An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol 53(Pt 5):427–433. doi:10.1099/jmm.0.05472-0
Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, Guimaraes V, Rabot S, Alcocer-Gonzalez JM, Gratadoux JJ, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, Gruss A, Langella P (2005) A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol 175(11):7297–7302. doi:10.4049/jimmunol.175.11.7297
Bierne H, Sabet C, Personnic N, Cossart P (2007) Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect 9(10):1156–1166. doi:10.1016/j.micinf.2007.05.003
Bines JE (2005) Rotavirus vaccines and intussusception risk. Curr Opin Gastroenterol 21(1):20–25
Bosma T, Kanninga R, Neef J, Audouy SAL, van Roosmalen ML, Steen A, Buist G, Kok J, Kuipers OP, Robillard G, Leenhouts K (2006) Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl Environ Microbiol 72(1):880–889. doi:10.1128/Aem.72.1.880-889.2006
Brinkman AB, Bell SD, Lebbink RJ, de Vos WM, van der Oost J (2002) The Sulfolobus solfataricus Lrp-like protein LysM regulates lysine biosynthesis in response to lysine availability. J Biol Chem 277(33):29537–29549. doi:10.1074/jbc.M203528200
Brinster S, Furlan S, Serror P (2007) C-terminal WxL domain mediates cell wall binding in Enterococcus faecalis and other gram-positive bacteria. J Bacteriol 189(4):1244–1253. doi:10.1128/Jb.00773-06
Buist G, Karsens H, Nauta A, van Sinderen D, Venema G, Kok J (1997) Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol 63(7):2722–2728
Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G, Haandrikman AJ (1995) Molecular-cloning and nucleotide-sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell-separation. J Bacteriol 177(6):1554–1563
Buist G, Ridder AN, Kok J, Kuipers OP (2006) Different subcellular locations of secretome components of Gram-positive bacteria. Microbiology 152(Pt 10):2867–2874. doi:10.1099/mic.0.29113-0
Buist G, Steen A, Kok J, Kuipers OP (2008) LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 68(4):838–847. doi:10.1111/j.1365-2958.2008.06211.x
Charbit A, Boulain JC, Ryter A, Hofnung M (1986) Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope; expression at the cell surface. EMBO J 5(11):3029–3037
Chen W, Georgiou G (2002) Cell-surface display of heterologous proteins: from high-throughput screening to environmental applications. Biotechnol Bioeng 79(5):496–503. doi:10.1002/bit.10407
Chen X, Chen Y, Li X, Chen N, Fang W (2009) Characterization of surface layer proteins in Lactobacillus crispatus isolate ZJ001. J Microbiol Biotechnol 19(10):1176–1183
Chen X, Choudhari SP, Kumar P, Toth RT, Kim JH, Van Roosmalen ML, Leenhouts K, Middaugh CR, Picking WL, Picking WD (2015) Biophysical characterization of the type III secretion system translocator proteins and the translocator proteins attached to bacterium-like particles. J Pharm Sci 104(12):4065–4073. doi:10.1002/jps.24659
Choudhari SP, Chen X, Kim JH, Van Roosmalen ML, Greenwood JC 2nd, Joshi SB, Picking WD, Leenhouts K, Middaugh CR, Picking WL (2015) Biophysical characterization of the type III secretion tip proteins and the tip proteins attached to bacterium-like particles. J Pharm Sci 104(2):424–432. doi:10.1002/jps.24047
Clemens JD, Harris JR, Khan MR, Kay BA, Yunus MD, Svennerholm AM, Sack DA, Chakraborty J, Stanton BF, Khan MU, Atkinson W, Holmgren J (1986) Field trial of oral cholera vaccines in Bangladesh. Lancet 2(8499):124–127
Clemens JD, Sack DA, Harris JR, Vanloon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, Stanton BF, Kay BA, Walter S, Eeckels R, Svennerholm AM, Holmgren J (1990) Field trial of oral cholera vaccines in Bangladesh—results from 3-year follow-up. Lancet 335(8684):270–273. doi:10.1016/0140-6736(90)90080-O
Daniel C, Roussel Y, Kleerebezem M, Pot B (2011) Recombinant lactic acid bacteria as mucosal biotherapeutic agents. Trends Biotechnol 29(10):499–508. doi:10.1016/j.tibtech.2011.05.002
de Haan A, Haijema BJ, Voorn P, Meijerhof T, van Roosmalen ML, Leenhouts K (2012) Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration. Vaccine 30(32):4884–4891. doi:10.1016/j.vaccine.2012.04.032
Dennehy PH (2007) Rotavirus vaccines—an update. Vaccine 25(16):3137–3141. doi:10.1016/j.vaccine.2007.01.102
Desvaux M, Dumas E, Chafsey I, Hebraud M (2006) Protein cell surface display in Gram-positive bacteria: from single protein to macromolecular protein structure. FEMS Microbiol Lett 256(1):1–15. doi:10.1111/j.1574-6968.2006.00122.x
Dieye Y, Usai S, Clier F, Gruss A, Piard JC (2001) Design of a protein-targeting system for lactic acid bacteria. J Bacteriol 183(14):4157–4166. doi:10.1128/JB.183.14.4157-4166.2001
Forrer P, Stumpp MT, Binz HK, Pluckthun A (2003) A novel strategy to design binding molecules harnessing the modular nature of repeat proteins. FEBS Lett 539(1–3):2–6. doi:10.1016/S0014-5793(03)00177-7
Galloway-Pena JR, Liang XW, Singh KV, Yadav P, Chang CY, La Rosa SL, Shelburne S, Ton-That H, Hook M, Murray BE (2015) The identification and functional characterization of WxL proteins from Enterococcus faecium reveal surface proteins involved in extracellular matrix interactions. J Bacteriol 197(5):882–892. doi:10.1128/Jb.02288-14
Gareau MG, Sherman PM, Walker WA (2010) Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7(9):503–514. doi:10.1038/nrgastro.2010.117
Garvey KJ, Saedi MS, Ito J (1986) Nucleotide sequence of Bacillus phage phi 29 genes 14 and 15: homology of gene 15 with other phage lysozymes. Nucleic Acids Res 14(24):10001–10008
Germanier R, Fuer E (1975) Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis 131(5):553–558
Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, Aylward RB (2009) Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J Infect Dis 200(5):794–801. doi:10.1086/605330
Gust AA, Willmann R, Desaki Y, Grabherr HM, Nurnberger T (2012) Plant LysM proteins: modules mediating symbiosis and immunity. Trends Plant Sci 17(8):495–502. doi:10.1016/j.tplants.2012.04.003
Ha S, Chang E, Lo MC, Men H, Park P, Ge M, Walker S (1999) The kinetic characterization of Escherichia coli MurG using synthetic substrate analogues. J Am Chem Soc 121(37):8415–8426. doi:10.1021/Ja991556t
Hagen KE, Guan LL, Tannock GW, Korver DR, Allison GE (2005) Detection, characterization, and in vitro and in vivo expression of genes encoding S-proteins in Lactobacillus gallinarum strains isolated from chicken crops. Appl Environ Microbiol 71(11):6633–6643. doi:10.1128/AEM.71.11.6633-6643.2005
Hang HC, Bertozzi CR (2001) Ketone isosteres of 2-N-acetamidosugars as substrates for metabolic cell surface engineering. J Am Chem Soc 123(6):1242–1243. doi:10.1021/Ja002962b
Heine SJ, Franco-Mahecha OL, Chen X, Choudhari S, Blackwelder WC, van Roosmalen ML, Leenhouts K, Picking WL, Pasetti MF (2015) Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol Cell Biol 93(7):641–652. doi:10.1038/icb.2015.24
Hitchcock SA, Eid CN, Aikins JA, Zia-Ebrahimi M, Blaszczak LC (1998) The first total synthesis of bacterial cell wall precursor UDP-N-acetylmuramyl-pentapeptide (Park nucleotide). J Am Chem Soc 120(8):1916–1917. doi:10.1021/Ja973172d
Hu SM, Kong J, Kong WT, Guo TT, Ji MJ (2010) Characterization of a novel LysM domain from Lactobacillus fermentum bacteriophage endolysin and its use as an anchor to display heterologous proteins on the surfaces of lactic acid bacteria. Appl Environ Microbiol 76(8):2410–2418. doi:10.1128/Aem.01752-09
Hu S, Kong J, Sun Z, Han L, Kong W, Yang P (2011) Heterologous protein display on the cell surface of lactic acid bacteria mediated by the s-layer protein. Microb Cell Factories 10:86. doi:10.1186/1475-2859-10-86
Hynonen U, Palva A (2013) Lactobacillus surface layer proteins: structure, function and applications. Appl Microbiol Biotechnol 97(12):5225–5243. doi:10.1007/s00253-013-4962-2
Ivanoff B, Levine MM, Lambert PH (1994) Vaccination against typhoid-fever—present status. Bull World Health Organ 72(6):957–971
Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR (2001) Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry 40(43):12864–12874. doi:10.1021/Bi010862s
Jakava-Viljanen M, Avall-Jaaskelainen S, Messner P, Sleytr UB, Palva A (2002) Isolation of three new surface layer protein genes (slp) from Lactobacillus brevis ATCC 14869 and characterization of the change in their expression under aerated and anaerobic conditions. J Bacteriol 184(24):6786–6795
Kalyanasundram J, Chia SL, Song AAL, Raha AR, Young HA, Yusoff K (2015) Surface display of glycosylated tyrosinase related protein-2 (TRP-2) tumour antigen on Lactococcus lactis. BMC Biotechnol 15. doi:10.1186/S12896-015-0231-Z
Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, Nishida H, Nagasaka K, Arimoto T, Yokoyama T, Wada-Hiraike O, Oda K, Sewaki T, Osuga Y, Fujii T (2014a) Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine 32(47):6233–6239. doi:10.1016/j.vaccine.2014.09.020
Keijzer C, Haijema BJ, Meijerhof T, Voorn P, de Haan A, Leenhouts K, van Roosmalen ML, van Eden W, Broere F (2014) Inactivated influenza vaccine adjuvanted with Bacterium-like particles induce systemic and mucosal influenza A virus specific T-cell and B-cell responses after nasal administration in a TLR2 dependent fashion. Vaccine 32(24):2904–2910. doi:10.1016/j.vaccine.2014.02.019
Kim SY, CG O, Lee YJ, Choi KH, Shin DS, Lee SK, Park KJ, Shin H, Park MS, Lee JH (2013) Sequence analysis of a cryptic plasmid pKW2124 from Weissella cibaria KLC140 and construction of a surface display vector. J Microbiol Biotechnol 23(4):545–554. doi:10.4014/jmb.1301.01018
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MWEJ, Stiekema W, Lankhorst RMK, Bron PA, Hoffer SM, Groot MNN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 100(4):1990–1995. doi:10.1073/pnas.0337704100
Kobierecka P, Wyszynska A, Maruszewska M, Wojtania A, Zylinska J, Bardowski J, Jagusztyn-Krynicka EK (2015) Lactic acid bacteria as a surface display platform for Campylobacter jejuni antigens. J Mol Microbiol Biotechnol 25(1):1–10. doi:10.1159/000368780
Kuipers OP, Beerthuyzen MM, de Ruyter PG, Luesink EJ, de Vos WM (1995) Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem 270(45):27299–27304
Le Loir Y, Azevedo V, Oliveira SC, Freitas DA, Miyoshi A, Bermudez-Humaran LG, Nouaille S, Ribeiro LA, Leclercq S, Gabriel JE, Guimaraes VD, Oliveira MN, Charlier C, Gautier M, Langella P (2005) Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb Cell Factories 4. doi:10.1186/1475-2859-4-2
Lee SY, Choi JH, ZH X (2003) Microbial cell-surface display. Trends Biotechnol 21(1):45–52
Leenhouts K, Buist G, Kok J (1999) Anchoring of proteins to lactic acid bacteria. Antonie Van Leeuwenhoek 76(1–4):367–376
Lin KH, Hsu AP, Shien JH, Chang TJ, Liao JW, Chen JR, Lin CF, Hsu WL (2012) Avian reovirus sigma C enhances the mucosal and systemic immune responses elicited by antigen-conjugated lactic acid bacteria. Vaccine 30(33):5019–5029. doi:10.1016/j.vaccine.2012.04.043
Liu DQ, Ge JW, Qiao XY, Jiang YP, Liu SM, Li YJ (2012a) High-level mucosal and systemic immune responses induced by oral administration with Lactobacillus-expressed porcine epidemic diarrhea virus (PEDV) S1 region combined with Lactobacillus-expressed N protein. Appl Microbiol Biotechnol 93(6):2437–2446. doi:10.1007/s00253-011-3734-0
Liu TT, Liu ZX, Song CJ, YF H, Han ZF, She J, Fan FF, Wang JW, Jin CW, Chang JB, Zhou JM, Chai JJ (2012b) Chitin-induced dimerization activates a plant immune receptor. Science 336(6085):1160–1164. doi:10.1126/science.1218867
Liu HT, Sadamoto R, Sears PS, Wong CH (2001) An efficient chemoenzymatic strategy for the synthesis of wild-type and vancomycin-resistant bacterial cell-wall precursors: UDP-N-acetylmuramyl-peptides. J Am Chem Soc 123(40):9916–9917. doi:10.1021/ja011708w
Liu W, Tan Z, Xue J, Luo W, Song H, Lv X, Zheng T, Xi T, Xing Y (2016) Therapeutic efficacy of oral immunization with a non-genetically modified Lactococcus lactis-based vaccine CUE-GEM induces local immunity against Helicobacter pylori infection. Appl Microbiol Biotechnol 100(14):6219–6229. doi:10.1007/s00253-016-7333-y
Low LY, Yang C, Perego M, Osterman A, Liddington R (2011) Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J Biol Chem 286(39):34391–34403. doi:10.1074/jbc.M111.244160
Mahal LK, Yarema KJ, Bertozzi CR (1997) Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 276(5315):1125–1128. doi:10.1126/science.276.5315.1125
Mann A, Friedrich N, Krarup A, Weber J, Stiegeler E, Dreier B, Pugach P, Robbiani M, Riedel T, Moehle K, Robinson JA, Rusert P, Pluckthun A, Trkola A (2013) Conformation-dependent recognition of HIV gp120 by designed ankyrin repeat proteins provides access to novel HIV entry inhibitors. J Virol 87(10):5868–5881. doi:10.1128/Jvi.00152-13
Mao R, Zhou K, Han Z, Wang Y (2016) Subtilisin QK-2: secretory expression in Lactococcus lactis and surface display onto gram-positive enhancer matrix (GEM) particles. Microb Cell Factories 15(1):80. doi:10.1186/s12934-016-0478-7
Masuda K, Kawata T (1980) Reassembly of the regularly arranged subunits in the cell-wall of Lactobacillus brevis and their reattachment to cell-walls. Microbiol Immunol 24(4):299–308
Masuda K, Kawata T (1981) Characterization of a regular array in the wall of Lactobacillus buchneri and its reattachment to the other wall components. J Gen Microbiol 124:81–90
Masuda K, Kawata T (1985) Reassembly of a regularly arranged protein in the cell-wall of Lactobacillus buchneri and its reattachment to cell-walls—chemical modification studies. Microbiol Immunol 29(10):927–938
Medina M, Villena J, Salva S, Vintini E, Langella P, Alvarez S (2008) Nasal administration of Lactococcus lactis improves local and systemic immune responses against Streptococcus pneumoniae. Microbiol Immunol 52(8):399–409. doi:10.1111/j.1348.0421.2008.00050.x
Men HB, Park P, Ge M, Walker S (1998) Substrate synthesis and activity assay for MurG. J Am Chem Soc 120(10):2484–2485. doi:10.1021/Ja974221p
Michon C, Langella P, Eijsink VG, Mathiesen G, Chatel JM (2016) Display of recombinant proteins at the surface of lactic acid bacteria: strategies and applications. Microb Cell Factories 15(1):70. doi:10.1186/s12934-016-0468-9
Miyoshi A, Poquet I, Azevedo V, Commissaire J, Bermudez-Humaran L, Domakova E, Le Loir Y, Oliveira SC, Gruss A, Langella P (2002) Controlled production of stable heterologous proteins in Lactococcus lactis. Appl Environ Microbiol 68(6):3141–3146. doi:10.1128/Aem.68.6.3141-3146.2002
Moeini H, Rahim RA, Omar AR, Shafee N, Yusoff K (2011) Lactobacillus acidophilus as a live vehicle for oral immunization against chicken anemia virus. Appl Microbiol Biotechnol 90(1):77–88. doi:10.1007/s00253-010-3050-0
Moorthy G, Ramasamy R (2007) Mucosal immunisation of mice with malaria protein on lactic acid bacterial cell walls. Vaccine 25(18):3636–3645. doi:10.1016/j.vaccine.2007.01.070
Morita H, Toh H, Oshima K, Murakami M, Taylor TD, Igimi S, Hattori M (2009) Complete genome sequence of the probiotic Lactobacillus rhamnosus ATCC 53103. J Bacteriol 191(24):7630–7631. doi:10.1128/JB.01287-09
Mota RM, Moreira JLS, Souza MR, Horta MF, Teixeira SMR, Neumann E, Nicoli JR, Nunes AC (2006) Genetic transformation of novel isolates of chicken Lactobacillus bearing probiotic features for expression of heterologous proteins: a tool to develop live oral vaccines. BMC Biotechnol 6. doi:10.1186/1472-6750-6-2
Narita J, Okano K, Kitao T, Ishida S, Sewaki T, Sung MH, Fukuda H, Kondo A (2006) Display of alpha-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl Environ Microbiol 72(1):269–275. doi:10.1128/AEM.72.1.269-275.2006
Nganou-Makamdop K, van Roosmalen ML, Audouy SAL, van Gemert GJ, Leenhouts K, Hermsen CC, Sauerwein RW (2012) Bacterium-like particles as multi-epitope delivery platform for Plasmodium berghei circumsporozoite protein induce complete protection against malaria in mice. Malar J 11. doi:10.1186/1475-2875-11-50
Ohnuma T, Onaga S, Murata K, Taira T, Katoh E (2008) LysM domains from Pteris ryukyuensis chitinase-A: a stability study and characterization of the chitin-binding site. J Biol Chem 283(8):5178–5187. doi:10.1074/jbc.M707156200
Okano K, Zhang Q, Kimura S, Narita J, Tanaka T, Fukuda H, Kondo A (2008) System using tandem repeats of the cA peptidoglycan-binding domain from Lactococcus lactis for display of both N- and C-terminal fusions on cell surfaces of lactic acid bacteria. Appl Environ Microbiol 74(4):1117–1123. doi:10.1128/Aem.02012-07
Pavot V, Rochereau N, Genin C, Verrier B, Paul S (2012) New insights in mucosal vaccine development. Vaccine 30(2):142–154. doi:10.1016/j.vaccine.2011.11.003
Perez CA, Eichwald C, Burrone O, Mendoza D (2005) Rotavirus vp7 antigen produced by Lactococcus lactis induces neutralizing antibodies in mice. J Appl Microbiol 99(5):1158–1164. doi:10.1111/j.1365-2672.2005.02709.x
Peterbauer C, Maischberger T, Haltrich D (2011) Food-grade gene expression in lactic acid bacteria. Biotechnol J 6(9):1147–1161. doi:10.1002/biot.201100034
Pontes DS, de Azeyedo MSP, Chatel JM, Langella P, Azeyedo V, Miyoshi A (2011) Lactococcus lactis as a live vector: heterologous protein production and DNA delivery systems. Protein Expr Purif 79(2):165–175. doi:10.1016/j.pep.2011.06.005
Pouwels PH, Leer RJ, Shaw M, den Bak-Glashouwer MJH, Tielen FD, Smit E, Martinez B, Jore J, Conway PL (1998) Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int J Food Microbiol 41(2):155–167. doi:10.1016/S0168-1605(98)00048-8
Qin JY, Wang XW, Kong J, Ma CQ, Xu P (2014) Construction of a food-grade cell surface display system for Lactobacillus casei. Microbiol Res 169(9–10):733–740. doi:10.1016/j.micres.2014.02.001
Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM (2011) Impact of rotavirus vaccination on diarrhea-related hospitalizations among children <5 years of age in Mexico. Pediatr Infect Dis J 30(1):S11–S15. doi:10.1097/INF.0b013e3181fefb32
Raha AR, Varma NRS, Yusoff K, Ross E, Foo HL (2005) Cell surface display system for Lactococcus lactis: a novel development for oral vaccine. Appl Microbiol Biotechnol 68(1):75–81. doi:10.1007/s00253-004-1851-8
Ramasamy R, Yasawardena S, Zomer A, Venema G, Kok J, Leenhouts K (2006) Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations. Vaccine 24(18):3900–3908. doi:10.1016/j.vaccine.2006.02.040
Ramirez K, Ditamo Y, Rodriguez L, Picking WL, van Roosmalen ML, Leenhouts K, Pasetti MF (2010) Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunology 3(2):159–171. doi:10.1038/mi.2009.131
Ravnikar M, Strukelj B, Obermajer N, Lunder M, Berlec A (2010) Engineered lactic acid bacterium Lactococcus lactis capable of binding antibodies and tumor necrosis factor alpha. Appl Environ Microbiol 76(20):6928–6932. doi:10.1128/Aem.00190-10
Reveneau N, Geoffroy MC, Locht C, Chagnaud P, Mercenier A (2002) Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 20(13–14):1769–1777
Ribelles P, Benbouziane B, Langella P, Suarez JE, Bermudez-Humaran LG (2013) Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl Microbiol Biotechnol 97(3):1231–1239. doi:10.1007/s00253-012-4575-1
Rigter A, Widjaja I, Versantvoort H, Coenjaerts FEJ, Van Roosmalen M, Leenhouts K, Rottier PJM, Haijema BJ, de Haan CAM (2013) A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. Plos One 8(8) doi:ARTN e7107210.1371/journal.pone.0071072
Robinson K, Chamberlain LM, Schofield KM, Wells JM, LePage RWF (1997) Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol 15(7):653–657. doi:10.1038/Nbt0797-653
Roland KL, Tinge SA, Killeen KP, Kochi SK (2005) Recent advances in the development of live, attenuated bacterial vectors. Curr Opin Mol Ther 7(1):62–72
Sadamoto R, Matsubayashi T, Shimizu M, Ueda T, Koshida S, Koda T, Nishimura S (2008) Bacterial surface engineering utilizing glucosamine phosphate derivatives as cell wall precursor surrogates. Chemistry 14(33):10192–10195. doi:10.1002/chem.200801734
Sadamoto R, Niikura K, Monde K, Nishimura SI (2003) Cell wall engineering of living bacteria through biosynthesis. Recognition of Carbohydrates in Biological Systems Pt A: General Procedures 362:273–286
Sadamoto R, Niikura K, Sears PS, Liu HT, Wong CH, Suksomcheep A, Tomita F, Monde K, Nishimura SI (2002) Cell-wall engineering of living bacteria. J Am Chem Soc 124(31):9018–9019
Sadamoto R, Niikura K, Ueda T, Monde K, Fukuhara N, Nishimura SI (2004) Control of bacteria adhesion by cell-wall engineering. J Am Chem Soc 126(12):3755–3761. doi:10.1021/ja039391i
Saluja V, Visser MR, ter Veer W, van Roosmalen ML, Leenhouts K, Hinrichs WLJ, Huckriede A, Frijlink HW (2010a) Influenza antigen-sparing by immune stimulation with Gram-positive enhancer matrix (GEM) particles. Vaccine 28(50):7963–7969. doi:10.1016/j.vaccine.2010.09.066
Saluja V, Visser MR, van Roosmalen ML, Leenhouts K, Huckriede A, Hinrichs WLJ, Frijlink HW (2010b) Gastro-intestinal delivery of influenza subunit vaccine formulation adjuvanted with Gram-positive enhancer matrix (GEM) particles. Eur J Pharm Biopharm 76(3):470–474. doi:10.1016/j.ejpb.2010.08.003
Samuelson P, Gunneriusson E, Nygren PA, Stahl S (2002) Display of proteins on bacteria. J Biotechnol 96(2):129–154. doi:10.1016/S0168-1656(02)00043-3
Sanchez JL, Vasquez B, Begue RE, Meza R, Castellares G, Cabezas C, Watts DM, Svennerholm AM, Sadoff JC, Taylor DN (1994) Protective efficacy of oral whole-cell recombinant-B-subunit cholera vaccine in peruvian military recruits. Lancet 344(8932):1273–1276. doi:10.1016/S0140-6736(94)90755-2
Sanchez-Vallet A, Saleem-Batcha R, Kombrink A, Hansen G, Valkenburg DJ, Thomma BPHJ, Mesters JR (2013) Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. Elife 2 doi:ARTN e0079010.7554/eLife.00790
Saxon E, Bertozzi CR (2000) Cell surface engineering by a modified Staudinger reaction. Science 287(5460):2007–2010. doi:10.1126/science.287.5460.2007
Seegers JFML (2002) Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol 20(12):508–515. doi:10.1016/S0167-7799(02)02075-9
Siezen R, Boekhorst J, Muscariello L, Molenaar D, Renckens B, Kleerebezem M (2006) Lactobacillus plantarum gene clusters encoding putative cell-surface protein complexes for carbohydrate utilization are conserved in specific gram-positive bacteria. BMC Genomics 7. doi:10.1186/1471-2164-7-126
Simsek O, Sabanoglu S, Con AH, Karasu N, Akcelik M, Saris PEJ (2013) Immobilization of nisin producer Lactococcus lactis strains to chitin with surface-displayed chitin-binding domain. Appl Microbiol Biotechnol 97(10):4577–4587. doi:10.1007/s00253-013-4700-9
Smit E, Pouwels PH (2002) One repeat of the cell wall binding domain is sufficient for anchoring the Lactobacillus acidophilus surface layer protein. J Bacteriol 184(16):4617–4619. doi:10.1128/Jb.184.16.4617-4619.2002
Smit E, Jager D, Martinez B, Tielen FJ, Pouwels PH (2002) Structural and functional analysis of the S-layer protein crystallisation domain of Lactobacillus acidophilus ATCC 4356: evidence for protein-protein interaction of two subdomains. J Mol Biol 324(5):953–964. doi:10.1016/S0022-2836(02)01135-X
Smit E, Oling F, Demel R, Martinez B, Pouwels PH (2001) The S-layer protein of Lactobacillus acidophilus ATCC 4356: identification and characterisation of domains responsible for S-protein assembly and cell wall binding. J Mol Biol 305(2):245–257. doi:10.1006/jmbi.2000.4258
Song NN, Duc TN, van Oeffelen L, Muyldermans S, Peeters E, Charlier D (2013) Expanded target and cofactor repertoire for the transcriptional activator LysM from Sulfolobus. Nucleic Acids Res 41(5):2932–2949. doi:10.1093/nar/gkt021
Stahl S, Uhlen M (1997) Bacterial surface display: trends and progress. Trends Biotechnol 15(5):185–192. doi:10.1016/S0167-7799(97)01034-2
Steen A, Buist G, Horsburgh GJ, Venema G, Kuipers OP, Foster SJ, Kok J (2005) AcmA of Lactococcus lactis is an N-acetylglucosaminidase with an optimal number of LysM domains for proper functioning. Febs. Journal 272(11):2854–2868. doi:10.1111/j.1742-4658.2005.04706.x
Steen A, Buist G, Leenhouts KJ, El Khattabi M, Grijpstra F, Zomer AL, Venema G, Kuipers OP, Kok J (2003) Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J Biol Chem 278(26):23874–23881. doi:10.1074/jbc.M211055200
Steiner D, Forrer P, Pluckthun A (2008) Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J Mol Biol 382(5):1211–1227. doi:10.1016/j.jmb.2008.07.085
Stiles ME, Holzapfel WH (1997) Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol 36(1):1–29. doi:10.1016/S0168-1605(96)01233-0
Stumpp MT, Binz HK, Amstutz P (2008) DARPins: a new generation of protein therapeutics. Drug Discov Today 13(15–16):695–701. doi:10.1016/j.drudis.2008.04.013
Sun W, Curtiss R (2013) Rational considerations about development of live attenuated Yersinia pestis vaccines. Curr Pharm Biotechnol 14(10):878–886
Sun Z, Kong J, Hu S, Kong W, Lu W, Liu W (2013) Characterization of a S-layer protein from Lactobacillus crispatus K313 and the domains responsible for binding to cell wall and adherence to collagen. Appl Microbiol Biotechnol 97(5):1941–1952. doi:10.1007/s00253-012-4044-x
Tarahomjoo S, Katakura Y, Satoh E, Shioya S (2008) Bidirectional cell-surface anchoring function of C-terminal repeat region of peptidoglycan hydrolase of Lactococcus lactis, IL1403. J Biosci Bioeng 105(2):116–121. doi:10.1263/jbb.105.116
Toh H, Oshima K, Nakano A, Takahata M, Murakami M, Takaki T, Nishiyama H, Igimi S, Hattori M, Morita H (2013) Genomic adaptation of the Lactobacillus casei group. PLoS One 8(10):e75073. doi:10.1371/journal.pone.0075073
TonThat H, Faull KF, Schneewind O (1997) Anchor structure of staphylococcal surface proteins—a branched peptide that links the carboxyl terminus of proteins to the cell wall. J Biol Chem 272(35):22285–22292. doi:10.1074/jbc.272.35.22285
Trong ND, van Oeffelen L, Song NN, Hassanzadeh-Ghassabeh G, Muyldermans S, Charlier D, Peeters E (2013) The genome-wide binding profile of the Sulfolobus solfataricus transcription factor Ss-LrpB shows binding events beyond direct transcription regulation. BMC Genomics 14. doi:10.1186/1471-2164-14-828
Turner MS, Hafner LM, Walsh T, Giffard PM (2004) Identification and characterization of the novel LysM domain-containing surface protein Sep from Lactobacillus fermentum BR11 and its use as a peptide fusion partner in Lactobacillus and Lactococcus. Appl Environ Microbiol 70(6):3673–3680. doi:10.1128/aem.70.6.3673-3680.2004
Van Braeckel-Budimir N, Haijema BJ, Leenhouts K (2013) Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front Immunol 4:282. doi:10.3389/fimmu.2013.00282
van Roosmalen ML, Kanninga R, El Khattabi M, Neef J, Audouy S, Bosma T, Kuipers A, Post E, Steen A, Kok J, Buist G, Kuipers OP, Robillard G, Leenhouts K (2006) Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods 38(2):144–149. doi:10.1016/j.ymeth.2005.09.015
Vanasseldonk M, Rutten G, Oteman M, Siezen RJ, Devos WM, Simons G (1990) Cloning of Usp45, a gene encoding a secreted protein from Lactococcus lactis subsp Lactis Mg1363. Gene 95(1):155–160. doi:10.1016/0378-1119(90)90428-T
Varma NR, Toosa H, Foo HL, Alitheen NB, Nor Shamsudin M, Arbab AS, Yusoff K, Abdul Rahim R (2013) Display of the viral epitopes on Lactococcus lactis: a model for food grade vaccine against EV71. Biotechnol Res Int 2013:431315. doi:10.1155/2013/431315
Villena J, Medina M, Vintini E, Alvarez S (2008) Stimulation of respiratory immunity by oral administration of Lactococcus lactis. Can J Microbiol 54(8):630–638. doi:10.1139/W08-052
Visweswaran GR, Leenhouts K, van Roosmalen M, Kok J, Buist G (2014) Exploiting the peptidoglycan-binding motif, LysM, for medical and industrial applications. Appl Microbiol Biotechnol 98(10):4331–4345. doi:10.1007/s00253-014-5633-7
Visweswaran GRR, Steen A, Leenhouts K, Szeliga M, Ruban B, Hesseling-Meinders A, Dijkstra BW, Kuipers OP, Kok J, Buist G (2013) AcmD, a homolog of the major autolysin AcmA of Lactococcus lactis, binds to the cell wall and contributes to cell separation and autolysis. Plos One 8(8). doi:10.1371/journal.pone.0072167
Vonheijne G (1990) The signal peptide. J Membr Biol 115(3):195–201
Wang M, Gao Z, Zhang Y, Pan L (2016) Lactic acid bacteria as mucosal delivery vehicles: a realistic therapeutic option. Appl Microbiol Biotechnol 100(13):5691–5701. doi:10.1007/s00253-016-7557-x
Wang XY, Zhang XC, Zhou DS, Yang RF (2013) Live-attenuated Yersinia pestis vaccines. Expert Review of Vaccines 12(6):677–686. doi:10.1586/Erv.13.42
Wells JM, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6(5):349–362. doi:10.1038/nrmicro1840
Wernerus H, Stahl S (2004) Biotechnological applications for surface-engineered bacteria. Biotechnol Appl Biochem 40(Pt 3):209–228. doi:10.1042/BA20040014
Wieczorek AS, Martin VJJ (2010) Engineering the cell surface display of cohesins for assembly of cellulosome-inspired enzyme complexes on Lactococcus lactis. Microb Cell Factories 9. doi:10.1186/1475-2859-9-69
Wieczorek AS, Martin VJJ (2012) Effects of synthetic cohesin-containing scaffold protein architecture on binding dockerin-enzyme fusions on the surface of Lactococcus lactis. Microb Cell Factories 11. doi:10.1186/1475-2859-11-160
Xu W, Huang M, Zhang Y, Yi X, Dong W, Gao X, Jia C (2011) Novel surface display system for heterogonous proteins on Lactobacillus plantarum. Lett Appl Microbiol 53(6):641–648. doi:10.1111/j.1472-765X.2011.03160.x
Yen C, Guardado JAA, Alberto P, Araujo DSR, Mena C, Cuellar E, Nolasco JB, De Oliveira LH, Pastor D, Tate JE, Parashar UD, Patel MM (2011) Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 30(1):S6–S10. doi:10.1097/INF.0b013e3181fefa05
Zadravec P, Mavric A, Bogovic Matijasic B, Strukelj B, Berlec A (2014) Engineering BmpA as a carrier for surface display of IgG-binding domain on Lactococcus lactis. Protein Eng Des Sel 27(1):21–27. doi:10.1093/protein/gzt059
Zadravec P, Strukelj B, Berlec A (2015a) Heterologous surface display on lactic acid bacteria: non-GMO alternative? Bioengineered 6(3):179–183. doi:10.1080/21655979.2015.1040956
Zadravec P, Strukelj B, Berlec A (2015b) Improvement of LysM-mediated surface display of designed Ankyrin repeat proteins (DARPins) in recombinant and nonrecombinant strains of Lactococcus lactis and Lactobacillus species. Appl Environ Microbiol 81(6):2098–2106. doi:10.1128/Aem.03694-14
Zeng GC, Chen JB, Zhong LY, Wang R, Jiang LF, Cai JY, Yan L, Huang D, Chen CY, Chen ZW (2009) NSOM- and AFM-based nanotechnology elucidates nano-structural and atomic-force features of a Y. pestis V immunogen-containing particle vaccine capable of eliciting robust response. Proteomics 9(6):1538–1547. doi:10.1002/pmic.200800528
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program, no. 2011CB504800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain studies with human participants or animals performed by any of authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mao, R., Wu, D. & Wang, Y. Surface display on lactic acid bacteria without genetic modification: strategies and applications. Appl Microbiol Biotechnol 100, 9407–9421 (2016). https://doi.org/10.1007/s00253-016-7842-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7842-8