Abstract
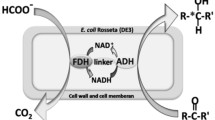
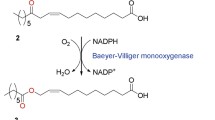
Enzyme fusion was investigated as a strategy to improve productivity of a two-step whole-cell biocatalysis. The biotransformation of long-chain sec-alcohols into esters by an alcohol dehydrogenase (ADH) and Baeyer–Villiger monooxygenases (BVMOs) was used as the model reaction. The recombinant Escherichia coli, expressing the fusion enzymes between the ADH of Micrococcus luteus NCTC2665 and the BVMO of Pseudomonas putida KT2440 or Rhodococcus jostii RHA1, showed significantly greater bioconversion activity with long-chain sec-alcohols (e.g., 12-hydroxyoctadec-9-enoic acid (1a), 13-hydroxyoctadec-9-enoic acid (2a), 14-hydroxyicos-11-enoic acid (4a)) when compared to the recombinant E. coli expressing the ADH and BVMOs independently. For instance, activity of the recombinant E. coli expressing the ADH-Gly-BVMO, in which glycine-rich peptide was used as the linker, with 1a was increased up to 22 μmol g dry cells−1 min−1. This value is over 40 % greater than the recombinant E. coli expressing the ADH and BVMO independently. The substantial improvement appeared to be driven by an increase in the functional expression of the BVMOs and/or an increase in mass transport efficiency by localizing two active sites in close proximity.



Similar content being viewed by others
References
Balke K, Kadow M, Mallin H, Sass S, Bornscheuer UT (2012) Discovery, application and protein engineering of Baeyer-Villiger monooxygenases for organic synthesis. Org Biomol Chem 10:6249–6265. doi:10.1039/c2ob25704a
Bisogno FR, Rioz-Martinez A, Rodriguez C, Lavandera I, de Gonzalo G, Pazmino DET, Fraaije MW, Gotor V (2010) Oxidoreductases working together: concurrent obtaining of valuable derivatives by employing the PIKAT method. ChemCatChem 2:946–949. doi:10.1002/cctc.201000115
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485:185–194. doi:10.1038/nature11117
Cheesman MJ, Kneller MB, Kelly EJ, Thompson SJ, Yeung CK, Eaton DL, Rettie AE (2001) Purification and characterization of hexahistidine-tagged cyclohexanone monooxygenase expressed in Saccharomyces cerevisiae and Escherichia coli. Protein Expr Purif 21:81–86. doi:10.1006/prep.2000.1340
Chen X, Zaro JL, Shen W-C (2013) Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev 64:1357–1369. doi:10.1016/j.addr.2012.09.039
de Gonzalo G, Mihovilovic MD, Fraaije MW (2010) Recent developments in the application of Baeyer-Villiger monooxygenases as biocatalysts. ChemBioChem 11:2208–2231. doi:10.1002/cbic.201000395
de Gonzalo G, Smit C, Jin J, Minnaard AJ, Fraaije MW (2011) Turning a riboflavin-binding protein into a self-sufficient monooxygenase by cofactor redesign. Chem Commun 47:11050–11052. doi:10.1039/c1cc14039f
Frese M, Guzowska PH, Voss H, Sewald N (2014) Regioselective enzymatic halogenation of substituted tryptophan derivatives using the FAD-dependent halogenase RebH. ChemCatChem 6:1270–1276. doi:10.1002/cctc.201301090
Gall M, Thomsen M, Peters C, Pavlidis IV, Jonczyk P, Gruenert PP, Beutel S, Scheper T, Gross E, Backes M, Geissler T, Ley JP, Hilmer J-M, Krammer G, Palm GJ, Hinrichs W, Bornscheuer UT (2013) Enzymatic conversion of flavonoids using bacterial chalcone isomerase and enoate reductase. Angew Chem Int Ed 53:1439–1442. doi:10.1002/anie.201306952
Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA III, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi:10.1038/nmeth.1318
Holtmann D, Fraaije MW, Arends IWCE, Opperman DJ, Hollmann F (2014) The taming of oxygen: biocatalytic oxyfunctionalisations. Chem Commun. doi:10.1039/c3cc49747j
Ishikawa M, Tsuchiya D, Oyama T, Tsunaka Y, Morikawa K (2004) Structural basis for channelling mechanism of a fatty acid beta-oxidation multienzyme complex. EMBO J 23:2745–2754. doi:10.1038/sj.emboj.7600298
Jang H-Y, Jeon E-Y, Baek AH, Lee S-M, Park J-B (2014) Production of ω-hydroxyundec-9-enoic acid and n-heptanoic acid from ricinoleic acid by recombinant Escherichia coli-based biocatalyst. Process Biochem 49:617–622. doi:10.1016/j.procbio.2014.01.025
Kirschner A, Altenbuchner J, Bornscheuer UT (2007) Cloning, expression, and characterization of a Baeyer-Villiger monooxygenase from Pseudomonas fluorescens DSM 50106 in E. coli. Appl Microbiol Biotechnol 73:1065–1072. doi:10.1007/s00253-006-0556-6
Ladkau N, Schmid A, Buehler B (2014) The microbial cell—functional unit for energy dependent multistep biocatalysis. Curr Opin Biotechnol 30:178–189. doi:10.1016/j.copbio.2014.06.003
Lee DH, Kim MD, Lee WH, Kweon DH, Seo JH (2004) Consortium of fold-catalyzing proteins increases soluble expression of cyclohexanone monooxygenase in recombinant Escherichia coli. Appl Microbiol Biotechnol 63:549–552. doi:10.1007/s00253-003-1370-z
Lee JW, Na D, Park JM, Lee J, Choi S, Lee SY (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 8:536–546. doi:10.1038/nchembio.970
Lin Y, Jain R, Yan Y (2014) Microbial production of antioxidant food ingredients via metabolic engineering. Curr Opin Biotechnol 26:71–78. doi:10.1016/j.copbio.2013.10.004
Lopez-Gallego F, Schmidt-Dannert C (2010) Multi-enzymatic synthesis. Curr Opin Chem Biol 14:174–183. doi:10.1016/j.cbpa.2009.11.023
Mallin H, Wulf H, Bornscheuer UT (2013) A self-sufficient Baeyer-Villiger biocatalysis system for the synthesis of ε-caprolactone from cyclohexanol. Enzym Microb Technol 53:283–287. doi:10.1016/j.enzmictec.2013.01.007
Niehaus JRWG, Frielle T, Kingsley JREA (1978) Purification and characterization of a secondary alcohol dehydrogenase from a pseudomonad. J Bacteriol 134:177–183
Oberleitner N, Peters C, Muschiol J, Kadow M, Sass S, Bayer T, Schaaf P, Iqbal N, Rudroff F, Mihovilovic MD, Bornscheuer UT (2013) An enzymatic toolbox for cascade reactions: a showcase for an in vivo redox sequence in asymmetric synthesis. ChemCatChem 5:3524–3528. doi:10.1002/cctc.201300604
Orru R, Dudek HM, Martinoli C, Pazmino DET, Royant A, Weik M, Fraaije MW, Mattevi A (2011) Snapshots of enzymatic Baeyer-Villiger catalysis: oxygen activation and intermediate stabilization. J Biol Chem 286:29284–29291. doi:10.1074/jbc.M111.255075
Pazmino DET, Snajdrova R, Baas B-J, Ghobrial M, Mihovilovic MD, Fraaije MW (2008) Self-sufficient Baeyer-Villiger monooxygenases: effective coenzyme regeneration for biooxygenation by fusion engineering. Angew Chem Int Ed 47:2275–2278. doi:10.1002/anie.200704630
Pazmino DET, Riebel A, de Lange J, Rudroff F, Mihovilovic MD, Fraaije MW (2009) Efficient biooxidations catalyzed by a new generation of self-sufficient Baeyer-Villiger monooxygenases. ChemBioChem 10:2595–2598. doi:10.1002/cbic.200900480
Rehdorf J, Kirschner A, Bornscheuer UT (2007) Cloning, expression and characterization of a Baeyer-Villiger monooxygenase from Pseudomonas putida KT2440. Biotechnol Lett 29:1393–1398. doi:10.1007/s10529-007-9401-y
Rioz-Martinez A, Bisogno FR, Rodriguez C, de Gonzalo G, Lavandera I, Pazmino DET, Fraaije MW, Gotor V (2010) Biocatalysed concurrent production of enantioenriched compounds through parallel interconnected kinetic asymmetric transformations. Org Biomol Chem 8:1431–1437. doi:10.1039/b925377g
Sattler JH, Fuchs M, Tauber K, Mutti FG, Faber K, Pfeffer J, Haas T, Kroutil W (2012) Redox self-sufficient biocatalyst network for the amination of primary alcohols. Angew Chem Int Ed 51:9156–9159. doi:10.1002/anie.201204683
Song JW, Jeon EY, Song DH, Jang HY, Bornscheuer UT, Oh DK, Park JB (2013) Multistep enzymatic synthesis of long-chain alpha, ω-dicarboxylic and ω-hydroxycarboxylic acids from renewable fatty acids and plant oils. Angew Chem Int Ed 52:2534–2537. doi:10.1002/anie.201209187
Song JW, Lee J-H, Bornscheuer UT, Park JB (2014) Microbial synthesis of medium chain α, ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long chain fatty acids. Adv Synth Catal 356:1782–1788. doi:10.1002/adsc.201300784
Staudt S, Bornscheuer UT, Menyes U, Hummel W, Groeger H (2013) Direct biocatalytic one-pot-transformation of cyclohexanol with molecular oxygen into ε-caprolactone. Enzym Microb Technol 53:288–292. doi:10.1016/j.enzmictec.2013.03.011
Szolkowy C, Eltis LD, Bruce NC, Grogan G (2009) Insights into sequence-activity relationships amongst Baeyer-Villiger monooxygenases as revealed by the intragenomic complement of enzymes from Rhodococcus jostii RHA1. ChemBioChem 10:1208–1217. doi:10.1002/cbic.200900011
van Beek HL, Wijma HJ, Fromont L, Janssen DB, Fraaije MW (2014) Stabilization of cyclohexanone monooxygenase by a computationally designed disulfide bond spanning only one residue. FEBS Open Bio 4:168–174. doi:10.1016/j.fob.2014.01.009
Zhang J, Yun J, Shang Z, Zhang X, Pan B (2009) Design and optimization of a linker for fusion protein construction. Prog Nat Sci 19:1197–1200. doi:10.1016/j.pnsc.2008.12.007
Zhao S, Kumar R, Sakai A, Vetting MW, Wood BM, Brown S, Bonanno JB, Hillerich BS, Seidel RD, Babbitt PC, Almo SC, Sweedler JV, Gerlt JA, Cronan JE, Jacobson MP (2013) Discovery of new enzymes and metabolic pathways by using structure and genome context. Nature 502(7473):698–702. doi:10.1038/nature12576
Acknowledgments
This study was supported by the Marine Biomaterials Research Center grant from Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 662 kb)
Rights and permissions
About this article
Cite this article
Jeon, EY., Baek, AH., Bornscheuer, U.T. et al. Enzyme fusion for whole-cell biotransformation of long-chain sec-alcohols into esters. Appl Microbiol Biotechnol 99, 6267–6275 (2015). https://doi.org/10.1007/s00253-015-6392-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6392-9