Abstract.
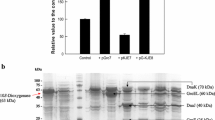
The cyclohexanone monooxygenase (CHMO) gene of Acinetobacter sp. NCIMB 9871 was simultaneously expressed with the genes encoding molecular chaperones and foldases in Escherichia coli. While the expression of the CHMO gene alone resulted in the formation of inclusion bodies, coexpression of the chaperone or foldase genes remarkably increased the production of soluble CHMO enzyme in recombinant E. coli. Furthermore, it was found that molecular chaperones were more beneficial than foldases for enhancing active CHMO enzyme production. The recombinant E. coli strain simultaneously expressing the genes for CHMO, GroEL/GroES and DnaK/DnaJ/GrpE showed a specific CHMO activity of 111 units g−1 cell protein, corresponding to a 38-fold enhancement in CHMO activity compared with the control E. coli strain expressing the CHMO gene alone.

Similar content being viewed by others
References
Altamirano MM, Garcia C, Possani LD, Fersht AR (1999) Oxidative refolding chromatography: folding of the scorpion toxin Cn5. Nat Biotechnol 17:187–191
Amrein KE, Takacs B, Steiger M, Molnos J, Flint NA, Burn P (1995) Purification and characterization of recombinant human p50csk protein-tyrosine kinase from and Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc Natl Acad Sci USA 92:1048–1052
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10: 411–421
Buckle AM, Zahn R, Fersht AR (1997) A structural model for GroEL-polypeptide recognition. Proc Natl Acad Sci USA 94:3571–3575
Cheesman MJ, Kneller MB, Kelly EJ, Thompson SJ, Yeung CK, Eaton DL, Rettie AE (2001) Purification and characterization of hexahistidine-tagged cyclohexanone monooxygenase expressed in Saccharomyces cerevisiae and Escherichia coli. Protein Expr Purif 21:81–86
Doig SD, Avenell PJ, Bird PA, Gallati P, Lander KS, Lye GJ, Wohlgemuth R, Woodley JM (2002) Reactor operation and scale-up of whole cell Baeyer–Villiger catalyzed lactone synthesis. Biotechnol Prog 18:1039–1046
Fernandez LA, Lorenzo V de (2001) Formation of disulphide bonds during secretion of proteins through the periplasmic-independent type I pathway. Mol Microbiol 40:332–346
Fischer CS, Yu C (2001) Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett 495:1–6
Jeong KJ, Lee SY (2000) Secretory production of human leptin in Escherichia coli. Biotechnol Bioeng 67:398–407
Jeong KJ, Lee SY (2003) Enhanced production of recombinant proteins in Escherichia coli by filamentation suppression. Appl Environ Microbiol 69:1295–1298
Jin HH, Han NS, Kweon DH, Park YC, Seo JH (2001) Effects of environmental factors of in vivo folding of Bacillus macerans cyclodextrin glycosyltransferase in recombinant Escherichia coli. J Microbiol Biotechnol 11:92–96
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 27:680–685
Maier R, Eckert B, Scholz C, Lilie H, Schmid FX (2003) Interaction of trigger factor with ribosome. J Mol Biol 326:585–592
Maskos K, Huber-Wunderlich M, Glockshuber R (2003). DsbA- and DsbC-catalyzed oxidative folding of proteins with complex disulfide bridge patterns in vitro and in vivo. J Mol Biol 325:495–513
Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T (1998) Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen Cryj2, in Escherichia coli. Appl Environ Microbiol 64:1694–1699
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Schlieker C, Bukau B, Mogk A (2002) Prevention and reversion of protein aggregation by molecular chaperones in E. coli cytosol: implications for their applicability in biotechnology. J Biotechnol 96:13–21
Shin CS, Hong MS, Kim DY, Shin HC, Lee J (1998) Growth-associated synthesis of recombinant human glucagon and human growth hormone in high-cell-density cultures of Escherichia coli. Appl Microbiol Biotechnol 49:364–370
Shin CS, Hong MS, Shin HC, Lee JW (2001) High-level production of recombinant human IFN-α2a with co-expression of tRNAArg(AGG/AGA) in high-cell-density cultures of Escherichia coli. Biotechnol Bioprocess Eng 6:301–305
Stewart JD (1998) Cyclohexanone monooxygenase: a useful reagent for asymmetric Bayer–Villiger reactions. Curr Org Chem 2:195–216
Thomas JG, Baneyx F (1996) Protein misfolding and inclusion body formation in recombinant Escherichia coli cells overexpressing heat-shock proteins. J Biol Chem 271:1141–1147
Thomas JG, Baneyx F (1997) Divergent effects of chaperone overexpression and ethanol supplementation on inclusion body formation in recombinant Escherichia coli. Protein Expr Purif 11:289–296
Trudgill PW (1990) Cyclohexanone 1,2-monooxygenase from Acinetobacter NCIMB 9871. Methods Enzymol 188:70–77
Walton AZ, Stewart JD (2002) An efficient enzymatic Baeyer–Villiger oxidation by engineered Escherichia coli cells under non-growing conditions. Biotechnol Prog 18:262–268
Willetts A (1997) Structural studies and synthetic applications of Baeyer–Villiger monooxygenases. Trends Biotechnol 15:55–62
Yang W, Zhang L, Lu Z, Tao W, Zhai Z (2001) A new method for protein coexpression in Escherichia coli using two incompatible plasmids. Protein Expr Purif 22:472–478
Zhang, Z, Li ZH, Wang F, Fang M, Yin CC, Zhou ZY, Lin Q, Huang HL (2002) Overexpression of DsbC and DsbG markedly improves soluble and functional expression of single-chain Fv antibodies in Escherichia coli. Protein Expr Purif 26:218–228
Acknowledgements.
We are grateful to Professor Jon D. Stewart (University of Florida, Gainesville, Fla.) for his kind donation of the plasmid pMM4. This work was supported by the Center for Advanced Bioseparation Technology and the Ministry of Education, through the BK21 program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, DH., Kim, MD., Lee, WH. et al. Consortium of fold-catalyzing proteins increases soluble expression of cyclohexanone monooxygenase in recombinant Escherichia coli . Appl Microbiol Biotechnol 63, 549–552 (2004). https://doi.org/10.1007/s00253-003-1370-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1370-z