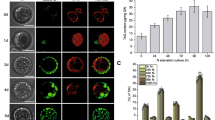
Abstract
Oleaginous Rhodococcus strains are able to accumulate large amounts of triacylglycerol (TAG). Phosphatidic acid phosphatase (PAP) enzyme catalyzes the dephosphorylation of phosphatidic acid (PA) to yield diacylglycerol (DAG), a key precursor for TAG biosynthesis. Studies to establish its role in lipid metabolism have been mainly focused in eukaryotes but not in bacteria. In this work, we identified and characterized a putative PAP type 2 (PAP2) encoded by the ro00075 gene in Rhodococcus jostii RHA1. Heterologous expression of ro00075 in Escherichia coli resulted in a fourfold increase in PAP activity and twofold in DAG content. The conditional deletion of ro00075 in RHA1 led to a decrease in the content of DAG and TAG, whereas its overexpression in both RHA1 and Rhodococcus opacus PD630 promoted an increase up to 10 to 15 % by cellular dry weight in TAG content. On the other hand, expression of ro00075 in the non-oleaginous strain Rhodococcus fascians F7 promoted an increase in total fatty acid content up to 7 % at the expense of free fatty acid (FFA), DAG, and TAG fractions. Moreover, co-expression of ro00075/atf2 genes resulted in a fourfold increase in total fatty acid content by a further increase of the FFA and TAG fractions. The results of this study suggest that ro00075 encodes for a PAP2 enzyme actively involved in TAG biosynthesis. Overexpression of this gene, as single one or with an atf gene, provides an alternative approach to increase the biosynthesis and accumulation of bacterial oils as a potential source of raw material for biofuel production.







Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Alvarez HM (2003) Relationship between β-oxidation pathway and the hydrocarbon-degrading profile in actinomycetes bacteria. Int Biodeter Biodegr 52:35–42
Alvarez HM (2010) Biotechnological production and significance of triacylglycerols and wax esters. In: Kenneth NT (ed) Microbiology of hydrocarbons, oils, lipids, and derived compounds. Springer, Heidelberg, pp 2995–3002
Alvarez HM, Steinbüchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376
Alvarez HM, Steinbüchel A (2010) Physiology biochemistry and molecular biology of triacylglycerol accumulation by Rhodococcus. In: Alvarez HM (ed) Biology of Rhodococcus. Microbiology monographs series. Springer, Heidelberg, pp 263–290
Alvarez HM, Mayer F, Fabritius D, Steinbüchel A (1996) Formation of intracytoplasmic lipid inclusion by Rhodococcus opacus PD630. Arch Microbiol 165:377–386
Alvarez HM, Kalscheuer R, Steinbüchel A (1997) Accumulation of storage lipids in species of Rhodococcus and Nocardia and effect of inhibitors and polyethylene glycol. Fett-Lipid 99:239–246
Alvarez HM, Kalscheuer R, Steinbüchel A (2000) Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl Microbiol Biotechnol 54(2):218–223
Alvarez AF, Alvarez HM, Kalscheuer R, Wältermann M, Steinbüchel A (2008) Cloning and characterization of a gene involved in triacylglycerol biosynthesis and identification of additional homologous genes in the oleaginous bacterium Rhodococcus opacus PD630. Microbiology 154:2327–2335
Alvarez HM, Silva RA, Herrero M, Hernández MA, Villalba MS (2013) Metabolism of triacylglycerols in Rhodococcus species insights from physiology and molecular genetics. J Mol Biochem 2:69–78
Barksdale L, Kim KS (1977) Mycobacterium. Bacteriol Rev 41:217–372
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Carman GM (1997) Phosphatidate phosphatases and diacylglycerol pyrophosphate phosphatases in Saccharomyces cerevisiae and Escherichia coli. Biochim Biophys Acta 1348:45–55
Carman GM, Han GS (2006) Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci 31(12):694–699
Chen Y, Ding Y, Yang L, Yu J, Liu G, Wang X, Zhang S, Yu D, Song L, Zhang H, Zhang C, Huo L, Huo C, Wang Y, Du Y, Zhang H, Zhang P, Na H, Xu S, Zhu Y, Xie Z, He T, Zhang Y, Wang G, Fan Z, Yang F, Liu H, Wang X, Zhang X, Zhang MQ, Li Y, Steinbüchel A, Fujimoto T, Cichello S, Yu J, Liu P (2013) Integrated omics study delineates the dynamics of lipid droplets in Rhodococcus opacus PD630. Nucleic Acids Res 42(2):1052–1064
Comba S, Menendez-Bravo S, Arabolaza A, Gramajo H (2013) Identification and physiological characterization of phosphatidic acid phosphatase enzymes involved in triacylglycerol biosynthesis in Streptomyces coelicolor. Microb Cell Fact 12:9
Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE (2004) Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030
Dillon DA, Wu WI, Riedel B, Wissing JB, Dowhan W, Carman GM (1996) The Escherichia coli pgpB gene encodes for a diacylglycerol pyrophosphate phosphatase activity. J Biol Chem 271:30548–30553
Ding Y, Yang L, Zhang S, Wang Y, Du Y, Pu J, Peng G, Chen Y, Zhang H, JinhaiYu J, Hang H, Wu P, Yang F, Yang H, Steinbüchel A, Liu P (2012) Identification of the major functional proteins of prokaryotic lipid droplets. J Lipid Res 53:399–411
Duncombe WG (1963) The colorimetric micro-determination of long chain fatty acids. Biochem J 88(1):7–10
Fan J, Jiang D, Zhao Y, Liu J, Zhang XC (2014) Crystal structure of lipid phosphatase Escherichia coli phosphatidylglycerophosphate phosphatase B. Proc Natl Acad Sci 111(21):7636–7640
Han GS, Wu WI, Carman GM (2006) The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem 281(14):9210–9218
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hernández MA, Mohn WW, Martínez E, Rost E, Alvarez AF, Alvarez HM (2008) Biosynthesis of storage compounds by Rhodococcus jostii RHA1 and global identification of genes involved in their metabolism. BMC Genomics 12:600
Hernández MA, Arabolaza A, Rodríguez E, Gramajo H, Alvarez HM (2013) The atf2 gene is involved in triacylglycerol biosynthesis and accumulation in the oleaginous Rhodococcus opacus PD630. Appl Microbiol Biotechnol 97:2119–2130
Hetzler S, Steinbüchel A (2013) Establishment of cellobiose utilization for lipid production in Rhodococcus opacus PD630. Appl Environ Microbiol 79(9):3122–3125
Hirofumi H, Gordon RS, Mohn WW (2010) Involvement of a novel ABC transporter and monoalkyl phthalate ester hydrolase in phthalate ester catabolism by Rhodococcus jostii RHA1. Appl Environ Microbiol 76(5):1516–1523
Holder JW, Ulrich JC, DeBono AC, Godfrey PA, Desjardins CA, Zucker J, Zeng Q, Leach ALB, Ghiviriga I, Dancel C, Abeel T, Gevers D, Kodira CD, Desany B, Affourtit JP, Birren BW, Sinskey AJ (2011) Comparative and functional genomics of Rhodococcus opacus PD630 for biofuels development. PLoS Genet 7(9):e1002219. doi:10.1371/journal.pgen.1002219
Janßen H, Steinbüchel A (2014) Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnology for Biofuels 7:7
Kalscheuer R, Arenskötter M, Steinbüchel A (1999) Establishment of a gene transfer system for Rhodococcus opacus PD630 based on electroporation and its application for recombinant biosynthesis of poly(3-hydroxyalkanoic acids). Appl Microbiol Biotechnol 52:508–515
Kennedy EP (1961) Biosynthesis of complex lipids. Fed Proc 20:934–940
Kocsis MG, Weselake RJ (1996) Phosphatidate phosphatases of mammals yeast and higher plants. Lipids 31:785–802
Kosa M, Ragauskas AJ (2011) Lipids from heterotrophic microbes: advances in metabolism research. Trends Biotechnol 29(2):53–61
Leman J (1997) Oleaginous microorganisms: an assessment of the potential. Adv Appl Microbiol 43:195–243
Li Q, Du W, Liu D (2008) Perspectives of microbial oils for biodiesel production. Appl Microbiol Biotechnol 80(5):749–756
Liang MH, Jiang JG (2013) Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog Lipid Res 52(4):395–408
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
MacEachran DP, Prophete ME, Sinskey AJ (2010) The Rhodococcus opacus PD630 heparin-binding hemagglutinin homolog TadA mediates lipid body formation. Appl Environ Microbiol 76(21):7217–7225
Nakamura Y, Tsuchiya M, Ohta H (2007) Plastidic phosphatidic acid phosphatases identified in a distinct subfamily of lipid phosphate phosphatases with prokaryotic origin. J Biol Chem 282(39):29013–29021
Nakashima N, Tamura T (2004) Isolation and characterization of a rolling-circle-type plasmid from Rhodococcus erythropolis and application of the plasmid to multiple-recombinant-protein expression. Appl Environ Microbiol 70:5557–5568
Olukoshi ER, Packter NM (1994) Importance of stored triacylglycerols in Streptomyces: possible carbon source for antibiotics. Microbiology 140:931–943
Parsons JB, Rock CO (2013) Bacterial lipids: metabolism and membrane homeostasis. Prog Lipid Res 52(3):249–276
Ratledge C (1989) Biotechnology of oils and fats. In: Ratledge C, Wilkinson SG (eds) Microbial lipids. Academic, London, pp 567–650
Rotering H, Raetz CR (1983) Appearance of monoglyceride and triglyceride in the cell envelope of Escherichia coli mutants defective in diglyceride kinase. J Biol Chem 258:8068–8073
Rucker J, Paul J, Pfeifer BA, Lee K (2013) Engineering E. coli for triglyceride accumulation through native and heterologous metabolic reactions. App Microbiol Biotechnol 97:2753–2759
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Schlegel HG, Kaltwasser H, Gottschalk G (1961) Ein Submersverfahren zur Kultur Wasserstoff oxydierender Bakterien Wachstumsphysiologische Untersuchungen. Arch Mikrobiol 38:209–222
Seto M, Kimbara K, Shimura M, Hatta T, Fukuda M, Yano K (1995) A novel transformation of polychlorinated biphenyls by Rhodococcus sp. strain RHA1. Appl Environ Microbiol 61(9):3353–3358
Sigal YJ, Mcdermott MI, Morris AJ (2005) Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem 387:281–293
Simon R, Priefer U, Puhler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nature Biotechnol 1:784–791
Stukey J, Carman GM (1997) Identification of a novel phosphatase sequence motif. Protein Sci 6:469–472
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic Acid Res 22:4673–4680
Toke DA, Bennett WL, Dillon DA, Wu WI, Chen X, Ostrander DB, Oshiro J, Cremesti A, Voelker DR, Fischl AS, Carman GM (1998a) Isolation and characterization of the Saccharomyces cerevisiae DPP1 gene encoding diacylglycerol pyrophosphate phosphatase. J Biol Chem 273:3278–3284
Toke DA, Bennett WL, Oshiro J, Wu WI, Voelker DR, Carman GM (1998b) Isolation and characterization of the Saccharomyces cerevisiae LPP1 gene encoding a Mg2+-independent phosphatidate phosphatase. J Biol Chem 273:14331–14338
Touze T, Blanot D, Mengin-Lecreulx D (2008) Substrate specificity and membrane topology of Escherichia coli PgpB an undecaprenyl pyrophosphate phosphatase. J Biol Chem 283:16573–16583
Triccas JA, Parish T, Britton WJ, Giquel B (1998) An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol Lett 167:151–156
Villalba MS, Alvarez HM (2014) Identification of a novel ATP-binding cassette transporter involved in long-chain fatty acid import and its role in triacylglycerol accumulation in Rhodococcus jostii RHA1. Microbiology 160(Pt 7):1523–1532
Villalba MS, Hernández MA, Silva RA, Alvarez HM (2013) Genome sequences of triacylglycerol metabolism in Rhodococcus as a platform for comparative genomics. Journal of Mol Biochem 2:94–105
Voss I, Steinbüchel A (2001) High cell density cultivation of Rhodococcus opacus for lipid production at a pilot-plant scale. Appl Microbiol Biotechnol 55(5):547–555
Wagner S, Klepsch MM, Schlegel S, Appel A, Draheim R, Tarry M, Hogbom M, Van Wijk KJ, Slotboom DJ, Persson JO, De Gier JW (2008) Tuning Escherichia coli for membrane protein overexpression. Proc Natl Acad Sci 105(38):14371–14376
Wältermann M, Luftmann H, Baumeister D, Kalscheuer R, Steinbüchel A (2000) Rhodococcus opacus PD630 as a source of high-value single cell oil? Isolation and characterization of triacylgycerols and other storage lipids. Microbiology 146:1143–1149
Wawrik B, Harriman BH (2010) Rapid colorimetric quantification of lipid from algal cultures. J Microbiol Methods 80(3):262–266
Wu H, Karanjikar M, San KY (2014) Metabolic engineering of Escherichia coli for efficient free fatty acid production from glycerol. Metab Eng 8:82–91
Zhang YM, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Zhang Y, Yang Z, Huang X, Peng J, Fei X, Gu S, Xie Y, Ji C, Mao Y (2008) Cloning, expression and characterization of a thermostable PAP2L2, a new member of the type-2 phosphatidic acid phosphatase family from Geobacillus toebii T-85. Biosci Biotechnol Biochem 72(12):3134–3141
Acknowledgments
The authors thank W.W. Mohn for providing both R. jostii RHA1 and pTip-QC2 vector. This study was financially supported by the SCyT of the University of Patagonia San Juan Bosco, the Agencia Comodoro Conocimiento (MCR), Oil m&s Company, Project PIP-CONICET Nro. 0764, Project PFIP CHU-25 (COFECyT), and Project PICT2012 Nro. 2031 (ANPCyT), Argentina. Alvarez H.M. is a career investigator and Hernández M.A. is a posdoctoral scholarship holder of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 124 kb)
Rights and permissions
About this article
Cite this article
Hernández, M.A., Comba, S., Arabolaza, A. et al. Overexpression of a phosphatidic acid phosphatase type 2 leads to an increase in triacylglycerol production in oleaginous Rhodococcus strains. Appl Microbiol Biotechnol 99, 2191–2207 (2015). https://doi.org/10.1007/s00253-014-6002-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6002-2