Abstract
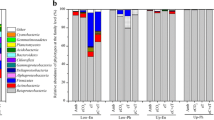
The phyllosphere, the aerial parts of terrestrial plants, represents the largest biological interface on Earth. This habitat is colonized by diverse microorganisms that affect plant health and growth. However, the community structure of these phyllosphere microorganisms and their responses to environmental changes, such as rising atmospheric CO2, are poorly understood. Using a massive parallel pyrosequencing technique, we investigated the feedback of a phyllosphere bacterial community in rice to elevated CO2 (eCO2) at the tillering, filling, and maturity stages under nitrogen fertilization with low (LN) and high application rates (HN). The results revealed 9,406 distinct operational taxonomic units that could be classified into 8 phyla, 13 classes, 26 orders, 59 families, and 120 genera. The family Enterobacteriaceae within Gammaproteobacteria was the most dominant phylotype during the rice growing season, accounting for 61.0–97.2 % of the total microbial communities. A statistical analysis indicated that the shift in structure and composition of phyllosphere bacterial communities was largely dependent on the rice growing stage. eCO2 showed a distinct effect on the structure of bacterial communities at different growth stages, and the most evident response of the community structure to eCO2 was observed at the filling stage. eCO2 significantly increased the relative abundance of the most dominant phylotype (Enterobacteriaceae) from 88.6 % at aCO2 (ambient CO2) to 97.2 % at eCO2 under LN fertilization at the filling stage, while it significantly decreased the total relative abundance of other phylotypes from 7.48 to 1.35 %. Similarly, higher value for the relative abundance of the most dominant family (Enterobacteriaceae) and lower value for the total relative abundance of other families were observed under eCO2 condition at other growth stages and under different N fertilizations, but the difference was not statistically significant. No consistent response pattern was observed along growth stages that could be attributed to N treatments. These results provide useful insights into our understanding of the response of a phyllosphere bacterial community to eCO2 with regards to the diversity, composition, and structure during rice growing seasons.




Similar content being viewed by others
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Leakey ADB, Ort DR, Long SP (2008) FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytol 179:5–9
Anten NPR, Hirose T, Onoda Y, Kinugasa T, Kim HY, Okada M, Kobayashi K (2004) Elevated CO2 and nitrogen availability have interactive effects on canopy carbon gain in rice. New Phytol 16:459–471
Austin EE, Castro HF, Sides KE, Schadt CW, Classen AT (2009) Assessment of 10 years of CO2 fumigation on soil microbial communities and function in a sweetgum plantation. Soil Biol Biochem 41:514–520
Badger MR, Bek EJ (2008) Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot 59:1525–1541
Bodenhausen N, Horton MW, Bergelson J (2013) Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS One 8:e56329
Brandl MT, Lindow SE (1998) Contribution of indole-3-acetic acid production to the epiphytic fitness of Erwinia herbicola. Appl Environ Microbiol 64:3256–3263
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Chinnadurai C, Balachandar D, Sundaram SP (2009) Characterization of 1-aminocyclopropane-1-carboxylate deaminase producing methylobacteria from phyllosphere of rice and their role in ethylene regulation. World J Microbiol Biotechnol 25:1403–1411
Costa DM, Samarasinghe SST, Dias HRD, Dissanayake DMN (2008) Control of rice sheath blight by phyllosphere epiphytic microbial antagonists. Phytoparasitica 36:52–65
De Maayer P, Chan WY, Blom J, Venter SN, Duffy B, Smits THM, Coutinho TA (2012) The large universal Pantoea plasmid LPP-1 plays a major role in biological and ecological diversification. BMC Genomics 13:625
Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt JA (2009) Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci U S A 106:16428–16433
Drissner D, Blum H, Tscherko D, Kandeler E (2007) Nine years of enriched CO2 changes the function and structural diversity of soil microorganisms in a grassland. Eur J Soil Sci 58:260–269
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Ellis RJ, Thompson IP, Bailey MJ (1999) Temporal fluctuations in the pseudomonad population associated with sugar beet leaves. FEMS Microbiol Ecol 28:345–356
Ercolani G (1991) Distribution of epiphytic bacteria on olive leaves and the influence of leaf age and sampling time. Microb Ecol 21:35–48
Feng YJ, Shen DL, Dong XZ, Song W (2003) In vitro symplasmata formation in the rice diazotrophic endophyte Pantoea agglomerans YS19. Plant Soil 255:435–444
Fierer N, Hamady M, Lauber CL, Knight R (2008) The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A 105:17994–17999
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017
Finkel OM, Burch AY, Elad T, Huse SM, Lindow SE, Post AF, Belkin S (2012) Distance-decay relationships partially determine diversity patterns of phyllosphere bacteria on Tamarix trees across the Sonoran Desert. Appl Environ Microbiol 78:6187–6193
Fisher RA (1930) The genetical theory of natural selection. Clarendon, Oxford
Freiberg E (1998) Microclimatic parameters influencing nitrogen fixation in the phyllosphere in a Costa Rican premontane rain forest. Oecologia 117(1):9–18
Ge Y, Chen CR, Xu ZH, Oren R, He JZ (2010) The spatial factor, rather than elevated CO2, controls the soil bacterial community in a temperate forest ecosystem. Appl Environ Microbiol 76:7429–7436
Goatley JL, Lewis RW (1966) Composition of guttation fluids from rye, wheat, and barley seedlings. Plant Physiol 41:373–375
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Gyaneshwar P, James EK, Mathan N, Reddy PM, Reinhold-Hurek B, Ladha JK (2001) Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J Bacteriol 183:2634–2645
Hartmann A, Schmid M, Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
He ZL, Xu MY, Deng Y, Kang S, Kellogg L, Wu LY, Van Nostrand JD, Hobbie SE, Reich PB, Zhou JZ (2010) Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2. Ecol Lett 13:564–575
Hunter PJ, Hand P, Pink D, Whipps JM, Bending GD (2010) Both leaf properties and microbe–microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce (Lactuca species) phyllosphere. Appl Environ Microbiol 76:8117–8125
Idso KE, Idso SB (1994) Plant responses to atmospheric CO2 enrichment in the face of environmental constraints: a review of the past 10 years’ research. Agric For Meteorol 69:153–203
Jackson C, Denney W (2011) Annual and seasonal variation in the phyllosphere bacterial community associated with leaves of the Southern Magnolia (Magnolia grandiflora). Microb Ecol 61:113–122
Kim HY, Lieffering M, Miura S, Kobayashi K, Okada M (2001) Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytol 150:223–229
Koops HP, Böttcher B, Möller UC, Pommerening-Röser A, Stehr G (1991) Classification of eight new species of ammonia-oxidizing bacteria: Nitrosomonas communis sp. nov., Nitrosomonas ureae sp. nov., Nitrosomonas aestuarii sp. nov., Nitrosomonas marina sp. nov., Nitrosomonas nitrosa sp. nov., Nitrosomonas eutropha sp. nov., Nitrosomonas oligotropha sp. nov. and Nitrosomonas halophila sp. nov. J Gen Microbiol 137:1689–1699
Lambais MR, Crowley DE, Cury JC, Büll RC, Rodrigues RR (2006) Bacterial diversity in tree canopies of the Atlantic forest. Science (New York, NY) 312:1917
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883
Lipson DA, Blair M, Barron-Gafford G, Grieve K, Murthy R (2006) Relationships between microbial community structure and soil processes under elevated atmospheric carbon dioxide. Microb Ecol 51:302–314
Liu G, Han Y, Zhu JG, Okada M, Nakamura H, Yoshimoto M (2002) Rice–wheat rotational FACE platform. I. System structure and control. Chin J Appl Ecol 13:1253–1258
Lopez-Velasco G, Welbaum GE, Boyer RR, Mane SP, Ponder MA (2011) Changes in spinach phylloepiphytic bacteria communities following minimal processing and refrigerated storage described using pyrosequencing of 16S rRNA amplicons. J Appl Microbiol 110:1203–1214
Miller TE, Werner PA (1987) Competitive effects and responses between plant species in a first-year old field community. Ecology 68:1201–1210
Mishra A, Chauhan P, Chaudhry V, Tripathi M, Nautiyal C (2011) Rhizosphere competent Pantoea agglomerans enhances maize (Zea mays) and chickpea (Cicer arietinum L.) growth, without altering the rhizosphere functional diversity. Antonie Van Leeuwenhoek 100:405–413
Montealegre CM, Van Kessel C, Blumenthal JM, Hur H-G, Hartwig UA, Sadowsky MJ (2000) Elevated atmospheric CO2 alters microbial population structure in a pasture ecosystem. Glob Chang Biol 6:475–482
Nakamura H, Tokida T, Yoshimoto M, Sakai H, Fukuoka M, Hasegawa T (2012) Performance of the enlarged rice-FACE system using pure CO2 installed in Tsukuba, Japan. J Agric Meteorol 68:15–63
Nix SS, Burpee LL, Jackson KL, Buck JW (2008) Short-term temporal dynamics of yeast abundance on the tall fescue phylloplane. Can J Microbiol 54:299–304
Okada M, Lieffering M, Nakamura H, Yoshimoto M, Kim HY, Kobayashi K (2001) Free-air CO2 enrichment (FACE) using pure CO2 injection: system description. New Phytol 150:251–260
Papen H, Geβler A, Zumbusch E, Rennenberg H (2002) Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Curr Microbiol 44:56–60
Peix A, Rivas R, Santa-Regina I, Mateos PF, Martínez-Molina E, Rodríguez-Barrueco C, Velázquez E (2004) Pseudomonas lutea sp. nov., a novel phosphate-solubilizing bacterium isolated from the rhizosphere of grasses. Int J Syst Evol Microbiol 54:847–850
Peñuelas J, Rico L, Ogaya R, Jump AS, Terradas J (2012) Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biol 14:565–575
Pusey PL, Stockwell VO, Reardon CL, Smits THM, Duffy B (2011) Antibiosis activity of Pantoea agglomerans biocontrol strain E325 against Erwinia amylovora on apple flower stigmas. Phytopathology 101:1234–1241
Quecine MC, Araújo WL, Rossetto PB, Ferreira A, Tsui S, Lacava PT, Mondin M, Azevedo JL, Pizzirani-Kleiner AA (2012) Sugarcane growth promotion by the endophytic bacterium Pantoea agglomerans 33.1. Appl Environ Microbiol 78:7511–7518
Rasche F, Marco-Noales E, Velvis H, van Overbeek LS, López MM, van Elsas JD, Sessitsch A (2006) Structural characteristics and plant-beneficial effects of bacteria colonizing the shoots of field grown conventional and genetically modified T4-lysozyme producing potatoes. Plant Soil 289:123–140
Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JHJ (2012) Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822
Redford AJ, Fierer N (2009) Bacterial succession on the leaf surface: a novel system for studying successional dynamics. Microb Ecol 58:189–198
Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N (2010) The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12:2885–2893
Reich PB, Hobbie SE (2013) Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat Clim Chang 3:278–282
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Ren SR, Zhu JG, Li HX, Wang XZ, Xie ZB, Zeng Q (2007) Effect of free-air CO2 enrichment (FACE) on microelements in paddy soil. Ecol Environ 16:982–986
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Roush ML, Radosevich SR (1985) Relationships between growth and competitiveness of four annual weeds. J Appl Ecol 22:895–905
Runion GB, Curl EA, Rogers HH, Backman PA, Rodríguez-Kábana R, Helms BE (1994) Effects of free-air CO2 enrichment on microbial populations in the rhizosphere and phyllosphere of cotton. Agric For Meteorol 70:117–130
Schu DJ, Carlier AL, Jamison KP, von Bodman S, Stevens AM (2009) Structure/function analysis of the Pantoea stewartii quorum-sensing regulator EsaR as an activator of transcription. J Bacteriol 191:7402–7409
Selvakumar G, Kundu S, Joshi P, Nazim S, Gupta A, Mishra P, Gupta H (2008) Characterization of a cold-tolerant plant growth-promoting bacterium Pantoea dispersa 1A isolated from a sub-alpine soil in the North Western Indian Himalayas. World J Microbiol Biotechnol 24:955–960
Shahzad S, Khalid A, Arshad M, Khalid M, Mehboob I (2008) Integrated use of plant growth promoting bacteria and P-enriched compost for improving growth, yield and nodulation of chickpea. Pak J Bot 40:1735–1741
Sievert SM, Kiene RP, Schulz-Vogt HN (2007) The sulfur cycle. Oceanography 20(2):117–123
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rrna sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Yang CH, Crowley DE, Borneman J, Keen NT (2001) Microbial phyllosphere populations are more complex than previously realized. Proc Natl Acad Sci U S A 98:3889–3894
Zavarzin GA, Stackebrandt E, Murray RG (1991) A correlation of phylogenetic diversity in the Proteobacteria with the influences of ecological forces. Can J Microbiol 37:1–6
Zhu CW, Zhu JG, Zeng Q, Liu G, Xie ZB, Tang HY, Cao JL, Zhao XZ (2009) Elevated CO2 accelerates flag leaf senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Funct Plant Biol 36:291–299
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (2010DFA22770), the National Science Foundation of China (41090281), Distinguished Young Scholar Programme of Jiangsu Province (BK2012048), and the Jiangsu Postdoctoral Science Foundation, China (1302031B). The authors thank Dr. Juan Zhou and Prof. Lianxing Yang from Yangzhou University and Chen Yan, Jing Xu, and Wanmeng Wang in our lab for help in sample collection. The authors would like to extend their gratitude to Dr. Haoye Tang and Dr. Qing Zeng for providing climate information, and Dr. Qiong Wang from Michigan State University for bioinformatic assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 458 kb)
Rights and permissions
About this article
Cite this article
Ren, G., Zhang, H., Lin, X. et al. Response of phyllosphere bacterial communities to elevated CO2 during rice growing season. Appl Microbiol Biotechnol 98, 9459–9471 (2014). https://doi.org/10.1007/s00253-014-5915-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5915-0