Abstract
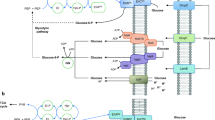
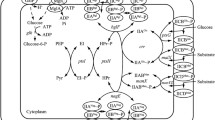
In Escherichia coli, the phosphoenolpyruvate–carbohydrate phosphotransferase system (PTS) is responsible for the transport and phosphorylation of sugars, such as glucose. PTS activity has a crucial role in the global signaling system that controls the preferential consumption of glucose over other carbon sources. When the cell is exposed to carbohydrate mixtures, the PTS prevents the expression of catabolic genes and activity of non-PTS sugars transport systems by carbon catabolite repression (CCR). This process defines some metabolic and physiological constraints that must be considered during the development of production strains. In this review, we summarize the importance of the PTS in controlling and influencing both PTS and non-PTS sugar transport processes as well as the mechanisms of transcriptional control involved in the expression of catabolic genes of non-PTS sugars in E. coli. We discuss three main approaches applied efficiently to avoid these constraints resulting in obtaining PTS− glc+ mutants useful for production purposes: (1) adaptive selection in chemostat culture system of PTS− mutants, resulting in the selection of strains that recovered the ability to grow in glucose, along with the simultaneous consumption of two carbon sources and reduced acetate production; (2) replacement in PTS− strains of the native GalP promoter by strong promoters or the substitution of this permease by recombinant glucose transport system; and (3) enhancement of Crp (crp+) in mgsA, pgi, and ptsG mutants, resulting in derivative strains that abolished CCR, allowing the simultaneous consumption of mixtures of sugars with low acetate production.


Similar content being viewed by others
References
Adler J, Epstein W (1974) Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci USA 71:2895–2899
Amster-Choder O (2005) The bgl sensory system: a transmembrane signaling pathway controlling transcriptional antitermination. Curr Opin Microbiol 8:127–134
Báez JL, Bolívar F, Gosset G (2001) Determination of 3-deoxy-d-arabino-heptulosonate 7-phosphate productivity and yield from glucose in Escherichia coli devoid of the glucose phosphotransferase transport system. Biotechnol Bioeng 73:530–535
Báez-Viveros JL, Osuna J, Hernández-Chávez G, Soberón X, Bolívar F, Gosset G (2004) Metabolic engineering and protein directed evolution increase the yield of l-phenylalanine synthesized from glucose in Escherichia coli. Biotechnol Bioeng 87:516–524
Báez-Viveros JL, Flores N, Suarez K, Castillo-España P, Bolivar F, Gosset G (2007) Metabolic transcription analysis of engineered Escherichia coli strains that overproduce l-phenylalanine. Microb Cell Fact 6:30
Bahr T, Lüttmann D, März RB, Görke B (2011) Insight into bacterial phosphotransferase system-mediated signaling by interspecies transplantation of a transcriptional regulator. J Bacteriol 193:2013–2026
Balderas-Hernandez VE, Sabido-Ramos A, Silva P, Cabrera-Valladares N, Hernandez-Chavez G, Baez-Viveros JL, Martinez A, Bolivar F, Gosset G (2009) Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb Cell Fact 8:19
Balderas-Hernandez VE, Hernandez-Montalvo V, Bolivar F, Gosset G, Martinez A (2011) Adaptive evolution of Escherichia coli inactivated in the phosphotransferase system operon improves co-utilization of xylose and glucose under anaerobic conditions. Appl Biochem Biotechnol 163:485–496
Balsalobre C, Johansson J, Uhlin BE (2006) Cyclic AMP-dependent osmoregulation of crp gene expression in Escherichia coli. J Bacteriol 188:5935–5944
Brückner R, Titgemeyer F (2002) Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209:141–148
Chandran SS, Yi J, Draths KM, von Daeniken R, Weber W, Frost JW (2003) Phosphoenolpyruvate availability and the biosynthesis of shikimic acid. Biotechnol Prog 19:808–814
Chávez-Béjar MI, Lara AR, López H, Hernández-Chávez G, Martinez A, Ramírez OT, Bolívar F, Gosset G (2008) Metabolic engineering of Escherichia coli for l-tyrosine production by expression of genes coding for the chorismate mutase domain of the native chorismate mutaseprephenate dehydratase and a cyclohexadienyl dehydrogenase from Zymomonas mobilis. Appl Environ Microbiol 74:3284–3290
De Anda R, Lara AR, Hernández V, Hernández-Montalvo V, Gosset G, Bolívar F, Ramírez OT (2006) Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab Eng 8:281–290
Death A, Ferenci T (1994) Between feast and famine: endogenous inducer synthesis in the adaptation of Escherichia coli to growth with limiting carbohydrates. J Bacteriol 176:5101–5107
Decker K, Plumbridge J, Boos W (1998) Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol Microbiol 27:381–390
De Lay N, Gottesman S (2009) The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J Bacteriol 191:461–476
Deutscher J (2008) The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol 11:87–93
Deutscher J, Francke C, Postma PW (2006) How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in Bacteria. Microbiol Mol Biol Rev 70:939–1031
Dien BS, Nichols NN, Bothast RJ (2002) Fermentation of sugar mixtures using Escherichia coli catabolite repression mutants engineered for production of l-lactic acid. J Ind Microbiol Biotechnol 29:221–227
Escalante A, Calderón R, Valdivia A, De Anda R, Hernández G, Ramírez OT, Gosset G, Bolívar F (2010) Metabolic engineering for the production of shikimic acid in an evolved Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system. Microb Cell Fact 9:1–12
Ferenci T (2001) Hungry bacteria: definition and properties of a nutritional state. Environ Microbiol 3:605–611
Flores N, Xiao J, Berry A, Bolívar F, Valle F (1996) Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat Biotechnol 14:620–623
Flores S, Gosset G, Flores N, de Graaf AA, Bolívar F (2002) Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase systemby 13C labeling and NMR spectroscopy. Metab Eng 4:124–137
Flores N, de Anda R, Flores S, Escalante A, Hernández G, Martínez A, Ramírez OT, Gosset G, Bolívar F (2004) Role of pyruvate oxidase in Escherichia coli strains lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system. J Mol Microbiol Biotechnol 8:209–221
Flores N, Flores S, Escalante A, de Anda R, Leal L, Malpica R, Georgelis D, Gosset G, Bolívar F (2005a) Adaptation for fast growth on glucose by differential expression of central carbon metabolism and gal regulon in an Escherichia coli strain lacking the phophoenolpyruvate:carbohydrate phosphotransferase system. Metab Eng 7:70–87
Flores S, Flores N, de Anda R, González A, Escalante A, Sigala JC, Gosset G, Bolívar F (2005b) Nutrient scavenging stress response in an Escherichia coli strain lacking the phophpenolpyruvate:carbohydrate phosphotransferase system, as explored by gene expression profile analysis. J Mol Microbiol Biotechnol 10:51–63
Flores N, Leal L, Sigala JC, de Anda R, Escalante A, Martínez A, Ramírez OT, Gosset G, Bolivar F (2007) Growth recovery on glucose under aerobic conditions of an Escherichia coli strain carrying a phosphoenolpyruvate:carbohydrate phosphotransferase system deletion by inactivating arcA and overexpressing the genes coding for glucokinase and galactose permease. J Mol Microbiol Biotechnol 13:105–116
Gama-Castro S, Salgado H, Peralta-Gil M, Santos-Zavaleta A, Muñiz-Rascado L, Solano-Lira H, Jiménez-Jacinto V, Weiss V, García-Sotelo JS, López-Fuentes A, Porrón-Sotelo L, Alquicira-Hernández S, Medina-Rivera A, Martínez-Flores I, Alquicira-Hernández K, Martínez-Adame R, Bonavides-Martínez C, Miranda-Ríos J, Huerta A, Mendoza-Vargas A, Collado-Torres L, Taboada B, Vega-Alvarado L, Olvera M, Olvera L, Grande R, Morett E, Collado-Vides J (2010) RegulonDB (version 7.0): transcriptional regulation of Escherichia coli K-12 integrated within genetic sensory response units (Gensor Units). Nucl Acids Res. doi:10.1093/nar/gkq1110
Görke B, Rak B (1999) Catabolite control of Escherichia coli regulatory protein BglG activity by antagonistically acting phosphorylations. EMBO J 18:3370–3379
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:sugar phosphotransferase system. Microb Cell Fact 4:14
Gosset G (2009) Production of aromatic compounds in bacteria. Curr Opin Biotechnol 20:651–658
Gosset G, Yong-Xiao J, Berry A (1996) A direct comparison of approaches for increasing carbon flow to aromatic biosynthesis in Escherichia coli. J Ind Microbiol 17:47–52
Hosono K, Kakuda H, Ichihara S (1995) Decreasing accumulation of acetate in a rich medium by Escherichia coli on introduction of genes on a multicopy plasmid. Biosci Biotechnol Biochem 59:256–261
Jackson DW, Simecka JW, Romeo T (2002) Catabolite repression of Escherichia coli biofilm formation. J Bacteriol 184:3406–3410
Johansson J, Balsalobre C, Wang SY, Urbonaviciene J, Jin DJ, Sondén B, Uhlin BE (2000) Nucleoid proteins stimulate stringently controlled bacterial promoters: a link between the cAMP–CRP and the (p)ppGpp regulons in Escherichia coli. Cell 102:475–485
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358
Keseler IM, Collado-Vides J, Santos-Zavaleta A, Peralta-Gil M, Gama-Castro S, Muñiz-Rascado L, Bonavides-Martínez C, Paley S, Krummenacker M, Altman T, Kaipa P, Spaulding A, Pacheco J, Latendresse M, Fulcher C, Sarker M, Shearer AG, Mackie A, Paulsen I, Gunsalus RP, Karp PD (2011) EcoCyc: a comprehensive database of Escherichia coli biology. Nucl Acids Res 39:D583–D590
Kimata K, Inada T, Tagami H, Aiba H (1998) A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol Microbiol 29:1509–1519
Knop DR, Draths KM, Chandran SS, Barker JL, von Daeniken R, Weber W, Frost JW (2001) Hydroaromatic equilibration during biosynthesis of shikimic acid. J Am Chem Soc 123:10173–10182
Koo BM, Yoon MJ, Lee CR, Nam TW, Choe YJ, Jaffe H, Peterkofsky A, Seok Y-J (2004) A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J Biol Chem 279:31613–31621
Krämer M, Bongaerts J, Bovenberg R, Kremer S, Müller U, Orf S, Wubbolts M, Raeven L (2003) Metabolic engineering for microbial production of shikimic acid. Metab Eng 5:277–283
LaDucca RJ, Berry A, Chotani G, Dodge TC, Gosset G, Valle F, Liao JC, Yong-Xiao J, Power SD (1999) Metabolic pathway engineering of aromatic compounds. In: Demain AL, David JE (eds) Manual of industrial microbiology and biotechnology, 2nd edn. ASM Press, Washington, D. C., pp 605–615
Landis L, Xu J, Johnson RC (1999) The cAMP receptor protein CRP can function as an osmoregulator of transcription in Escherichia coli. Genes Dev 13:3081–3091
Lara AR, Caspeta L, Gosset G, Bolívar F, Ramírez OT (2008) Utility of an Escherichia coli strain engineered in the substrate uptake system for improved culture performance at high glucose and cell concentrations: an alternative to fed-batch cultures. Biotechnol Bioeng 99:893–901
Larson TJ, Ye SZ, Weissenborn DL, Hoffmann HJ, Schweizer H (1987) Purification and characterization of the repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K12. J Biol Chem 262:15869–15874
Lin EC (1976) Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol 30:535–578
Mao XJ, Huo YX, Buck M, Kolb A, Wang YP (2007) Interplay between CRP–cAMP and PII–Ntr systems forms novel regulatory network between carbon metabolism and nitrogen assimilation in Escherichia coli. Nucl Acids Res 35:1432–1440
Martínez K, de Anda R, Hernández G, Escalante A, Gosset G, Ramírez OT, Bolívar F (2008) Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system. Microb Cell Fact 7:1
Muñoz AJ, Hernández-Chavez G, de Anda R, Martínez A, Bolívar F, Gosset G (2011) Metabolic engineering of Escherichia coli for improving l-3,4-dihydroxyphenylalanine (l-DOPA) synthesis from glucose. J Ind Microbiol Biotechnol 38:1845–1852
Nam TW, Cho SH, Shin D, Kim JH, Jeong JY, Lee JH, Roe JH, Peterkofsky A, Kang SO, Ryu S, Seok YJ (2001) The Escherichia coli glucose transporter enzyme IICB(Glc) recruits the global repressor Mlc. EMBO J 20:491–498
Nam TW, Jung HI, An YJ, Park YH, Lee SH, Seok YJ, Cha SS (2008) Analyses of Mlc–IIBGlc interaction and a plausible molecular mechanism of Mlc inactivation by membrane sequestration. Proc Natl Acad Sci USA 105:3751–3756
Nichols NN, Dien BS, Bothast RJ (2001) Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Appl Microbiol Biotechnol 56:120–125
Nishino K, Senda Y, Yamaguchi A (2008) CRP regulator modulates multidrug resistance of Escherichia coli by repressing the mdtEF multidrug efflux genes. J Antibiot 61:120–127
Park Y‑H, Lee BR, Seok Y‑J, Peterkofsky A (2006) In vitro reconstitution of catabolite repression in Escherichia coli. J Biol Chem 281:6448–6454
Plumbridge J (2001) DNA binding sites for the Mlc and NagC proteins: regulation of nagE, encoding the N-acetylglucosamine-specific transporter in Escherichia coli. Nucl Acids Res 29:506–514
Plumbridge J (2002) Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr Opin Microbiol 5:187–193
Postma PW, Lengeler JW, Jacobson GR (1996) Phosphoenolpyruvate: Carbohydrate phosphotransferase systems. In: Neidhart FC (ed) Escherichia coli and Salmonella. Cellular and molecular biology. ASM Press, Washington, D. C., pp 1149–1174
Saier MH Jr, Ramseier TM, Reizer J (1996) Regulation of carbon utilization. In: Neidhart FC (ed) Escherichia coli and Salmonella. Cellular and molecular biology. ASM Press, Washington, D. C., pp 1325–1343
Schnetz K (1995) Silencing of Escherichia coli bgl promoter by flanking sequence elements. EMBO J 14:2545–2550
Schnetz K, Toloczyki C, Rak B (1987) Beta-glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol 169:2579–2590
Sigala JC, Flores S, FloresN AC, de Anda R, Gosset G, Bolivar F (2009) Acetate metabolism in Escherichia coli strains lacking phosphoenolpyruvate: carbohydrate phosphotransferase system; evidence of carbon recycling strategies and futile cycles. J Mol Microbiol Biotechnol 16:224–235
Sinha S, Cameron AD, Redfield RJ (2009) Sxy induces a CRP-S regulon in Escherichia coli. J Bacteriol 191:5180–5195
Tchieu JH, Norris V, Edwards JS, Saier MH Jr (2001) The complete phosphotranferase system in Escherichia coli. J Mol Microbiol Biotechnol 3:329–346
Vanderpool CK (2007) Physiological consequences of small RNA-mediated regulation of glucose-phosphate stress. Curr Opin Microbiol 10:146–1451
Vanderpool CK, Gottesman S (2004) Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol Microbiol 54:1076–1089
Weissenborn DL, Wittekindt N, Larson TJ (1992) Structure and regulation of the glpFK operon encoding glycerol diffusion facilitator and glycerol kinase of Escherichia coli K-12. J Biol Chem 267:6122–6131
Yamada M, Saier MH (1988) Positive and negative regulators for glucitol (gut) operon expression in Escherichia coli. J Mol Biol 203:569–583
Yao R, Hirose Y, Sarkar D, Nakahigashi K, Ye Q, Shimizu K (2011) Catabolic regulation analysis of Escherichia coli and its crp, mlc, mgsA, pgi and ptsG mutants. Microb Cell Fact 10:67
Zhang Z, Gosset G, Barabote R, Gonzalez CS, Cuevas WA, Saier MH (2005) Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J Bacteriol 187:980–990
Acknowledgments
This work was supported by CONACYT Sector Salud 126793, Ciencia Básica 105782 grants, DGAPA-PAPIIT UNAM IN224709, IN202611, and IN206812 grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escalante, A., Salinas Cervantes, A., Gosset, G. et al. Current knowledge of the Escherichia coli phosphoenolpyruvate–carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation. Appl Microbiol Biotechnol 94, 1483–1494 (2012). https://doi.org/10.1007/s00253-012-4101-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4101-5