Abstract
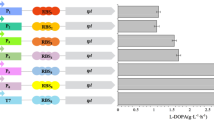
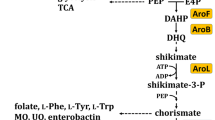
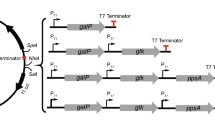
l-3,4-dihydroxyphenylalanine (l-DOPA) is an aromatic compound employed for the treatment of Parkinson's disease. Metabolic engineering was applied to generate Escherichia coli strains for the production of l-DOPA from glucose by modifying the phosphoenolpyruvate:sugar phosphotransferase system (PTS) and aromatic biosynthetic pathways. Carbon flow was directed to the biosynthesis of l-tyrosine (l-Tyr), an l-DOPA precursor, by transforming strains with compatible plasmids carrying genes encoding a feedback-inhibition resistant version of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase, transketolase, the chorismate mutase domain from chorismate mutase-prephenate dehydratase from E. coli and cyclohexadienyl dehydrogenase from Zymomonas mobilis. The effects on l-Tyr production of PTS inactivation (PTS− gluc+ phenotype), as well as inactivation of the regulatory protein TyrR, were evaluated. PTS inactivation caused a threefold increase in the specific rate of l-Tyr production (q l-Tyr), whereas inactivation of TyrR caused 1.7- and 1.9-fold increases in q l-Tyr in the PTS+ and the PTS− gluc+ strains, respectively. An 8.6-fold increase in l-Tyr yield from glucose was observed in the PTS− gluc+ tyrR − strain. Expression of hpaBC genes encoding the enzyme 4-hydroxyphenylacetate 3-hydroxylase from E. coli W in the strains modified for l-Tyr production caused the synthesis of l-DOPA. One of such strains, having the PTS− gluc+ tyrR − phenotype, displayed the best production parameters in minimal medium, with a specific rate of l-DOPA production of 13.6 mg/g/h, l-DOPA yield from glucose of 51.7 mg/g and a final l-DOPA titer of 320 mg/l. In a batch fermentor culture in rich medium this strain produced 1.51 g/l of l-DOPA in 50 h.


Similar content being viewed by others
References
Amann E, Ochs B, Abel KJ (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315
Báez JL, Bolívar F, Gosset G (2001) Determination of 3-deoxy-d-arabino-heptulosonate 7-phosphate productivity and yield from glucose in Escherichia coli devoid of the glucose phosphotransferase transport system. Biotechnol Bioeng 73:530–535
Balderas-Hernández VE, Sabido-Ramos A, Silva P, Cabrera-Valladares N, Hernández-Chávez G, Báez-Viveros JL, Martínez A, Bolívar F, Gosset G (2009) Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb Cell Fact 8:1–12
Chavez-Bejar MI, Lara AR, Lopez H, Hernandez-Chavez G, Martinez A, Ramirez OT, Bolivar F, Gosset G (2008) Metabolic engineering of Escherichia coli for l-tyrosine production by the expression of genes coding for the chorismate mutase domain of the native chorismate mutase-prephenate dehydratase and a cyclohexadienyl dehydrogenase from Zymomonas mobilis. Appl Environ Microbiol 74:3284–3290
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
De Anda R, Lara AR, Hernandez V, Hernandez-Montalvo V, Gosset G, Bolıvar F, Ramırez OT (2006) Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab Eng 8:281–290
Flores N, Yong-Xiao J, Berry A, Bolívar F, Valle F (1996) Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat Biotechnol 14:620–623
Flores S, Gosset G, Flores N, de Graff AA, Bolívar F (2002) Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase system by 13C labeling and NMR spectroscopy. Metab Eng 4:124–137
Förberg C, Eliaeson T, Häggström L (1988) Correlation of theoretical and experimental yields of phenylalanine from non-growing cells of a rec Escherichia coli strain. J Biotechnol 7:319–332
Gosset G, Yong-Xiao J, Berry A (1996) A direct comparison of approaches for increasing carbon flow to aromatic biosynthesis in Escherichia coli. J Ind Microbiol 17:47–52
Hernández-Montalvo V, Martínez A, Hernández-Chávez G, Bolivar F, Valle F, Gosset G (2003) Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol Bioeng 83:687–694
Ho PY, Chıou MS, Chao AC (2003) Production of l-DOPA by tyrosinase immobilized on modified polystyrene. Appl Biochem Biotechnol 111:139–152
Koyanagi T, Katayama T, Suzuki H, Nakazawa H, Yokozeki K, Kumagai H (2005) Effective production of 3, 4-dihydroxyphenyl l-alanine (l-dopa) with Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR. J Biotechnol 115:303–306
Kramer M, Kremer-Muschen S, Wubbolts MG (2006) Process for the preparation of l-3, 4-dihydroxyphenylalanine by aerobic fermentation of a microorganism. US Patent 2006/0141587A1
Krishnaveni R, Rathod V, Thakur MS, Neelgund YF (2009) Transformation of l-tyrosine to L-dopa by a novel fungus, Acremonium rutilum, under submerged fermentation. Curr Microbiol 58:122–128
Lee JY, Xun L (1998) Novel biological process for L-DOPA production from l-tyrosine by p-hydroxyphenylacetate 3-hydroxylase. Biotechnol Lett 20:479–482
Lee SG, Hong SP, Sung MH (1999) Development of an enzymatic system for the production of dopamine from catechol, pyruvate and ammonia. Enzyme Microb Technol 25:268–302
Lütke-Eversloh T, Stephanopoulos G (2007) l-Tyrosine production by deregulated strains of Escherichia coli. Appl Microbiol Biotechnol 75:103–110
Lütke-Eversloh T, Stephanopoulos G (2008) Combinatorial pathway analysis for improved l-tyrosine production in Escherichia coli: identification of enzymatic bottlenecks by systematic gene overexpression. Metab Eng 10:69–77
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring, NY, p 68
Patnaik R, Liao JC (1994) Engineering of Escherichia coli central metabolism for aromatic production with near theoretical yield. Appl Environ Microbiol 60:3903–3908
Patnaik R, Spitzer RG, Liao JC (1995) Pathway engineering for production of aromatics in Escherichia coli: confirmation of stoichiometric analysis by independent modulation of AroG, TktA, and Pps activities. Biotechnol Bioeng 46:361–370
Pittard J, Camakaris H, Yang J (2005) The TyrR regulon. Mol Microbiol 55:16–26
Prieto MA, Garcıa JL (1994) Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J Biol Chem 269:22823–22829
Qi WW, Vannelli T, Breinig S, Ben-Bassat A, Gatenby AA, Haynie SL, Sariaslani FS (2007) Functional expression of prokaryotic and eukaryotic genes in Escherichia coli for conversion of glucose to p-hydroxystyrene. Metab Eng 9:268–276
Reinhold DF, Utne T, Abramson NL (1987) Process for L-dopa. US patent 4716246
Snell KD, Draths KM, Frost JW (1996) Synthetic modification of the Escherichia coli chromosome: enhancing the biocatalytic conversion of glucose into aromatic chemicals. J Am Chem Soc 118:5605–5614
Takai A, Nishi R, Joe Y, Ito H (2005) l-Tyrosine producing bacterium and a method for producing l-tyrosine. US Patent application no. 2005/0277179A1
Acknowledgments
This work was supported by CONACyT grants 83039 and 126793. AJM was supported by a fellowship from CONACyT. We thank Luz María Martínez, Mercedes Enzaldo, Juan Manuel Hurtado, Mario Trejo and Martín Patiño for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz, A.J., Hernández-Chávez, G., de Anda, R. et al. Metabolic engineering of Escherichia coli for improving l-3,4-dihydroxyphenylalanine (l-DOPA) synthesis from glucose. J Ind Microbiol Biotechnol 38, 1845–1852 (2011). https://doi.org/10.1007/s10295-011-0973-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0973-0