Abstract
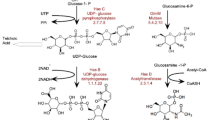
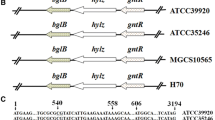
The has operon genes in the hyaluronan (HA) producer, Streptococcus zooepidemicus, encode for some of the critical enzymes in the HA biosynthetic pathway. Heterologous expression of different combinations of multiple has genes has resulted in increasing HA production to varying degrees in different recombinant strains. In this work, a recombinant Lactococcus lactis strain (SJR6) was constructed, with insertion of three has operon genes (hasABD) from S. zooepidemicus. The SJR6 strain was found to be a better HA producer than two previously constructed recombinant L. lactis strains (SJR2 and SJR3), containing hasAB and hasABC genes, respectively, but exhibited lower HA production than the native HA producer S. zooepidemicus. To understand the differences in HA yield between the various strains, transcriptions of the HA biosynthesis genes (has genes and their homologues) were compared at different phases of exponential growth of the L. lactis and S. zooepidemicus cultures. The mRNA levels of all the heterologous has genes were expectedly far higher than their corresponding homologues in the L. lactis strains. The relative mRNA level of the hasB-homologue, viz. ugd (encoding UDP-glucose dehydrogenase), was found to be much lower than that of other homologues, corroborating earlier reports which indicate tight transcriptional regulation of the ugd gene in L. lactis. Interestingly, all the has gene homologues were found to be up-regulated in all the recombinant L. lactis strains, when compared with the corresponding genes in the untransformed strain, L. lactis NZ9000. A transcription analysis of S. zooepidemicus cultures revealed that the has operon was down-regulated in the mid-exponential growth phase in comparison to the early- and late-exponential growth phases. The transcription analyses in this study have provided insights for the design of recombinant strains with higher HA productivity.








Similar content being viewed by others
References
Albertí S, Ashbaugh CD, Wessels MR (1998) Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol Microbiol 28:343–353
Bernish B, van de Rijn I (1999) Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem 274:4786–4793
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334
Blank LM, Hugenholtz P, Nielsen LK (2008) Evolution of the hyaluronic acid synthesis (has) operon in Streptococcus zooepidemicus and other pathogenic streptococci. J Mol Evol 67:13–22
Breborowicz A, Kuzlan-Pawlaczyk M, Wieczorowska-Tobis K, Wisniewska J, Tam P, French I, Wu G (1998) The effect of N-acetylglucosamine as a substrate for in vitro synthesis of glycosaminoglycans by human peritoneal mesothelial cells and fibroblasts. Adv Perit Dial 14:31–35
Carrier TA, Keasling JD (1997) Controlling messenger RNA stability in bacteria: strategies for engineering gene expression. Biotechnol Prog 13:699–708
Chen WY, Marcellin E, Hung J, Nielsen LK (2009) Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. J Biol Chem 284:18007–18014
Chien LJ, Lee CK (2007) Hyaluronic acid production by recombinant Lactococcus lactis. Appl Microbiol Biotechnol 77:339–349
Cho SP, Kim HG, Kim JG, Kim TH, Park SY (2004) Extraction method for high purity hyaluronic acid from rooster comb. World Patent: KR20040022760
Chong BF, Blank LM, McLaughin R, Nielsen LK (2005) Microbial hyaluronic acid production. Appl Microbiol Biotechnol 66:341–351
Clarkin CE, Allen S, Kuiper NJ, Wheeler BT, Wheeler-Jones CP, Pitsillides AA (2011) Regulation of UDP-glucose dehydrogenase is sufficient to modulate hyaluronan production and release, control sulfated GAG synthesis, and promote chondrogenesis. J Cell Physiol 226:749–761
Cole JN, Barnett TC, Nizet V, Walker MJ (2011) Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol 9:724–736
Crater DL, van de Rijn I (1995) Hyaluronic acid synthesis operon (has) expression in group A streptococci. J Biol Chem 270:18452–18458
Degeest B, de Vuyst L (2000) Correlation of activities of the enzymes a-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl Envioron Microbiol 66:3519–3527
Graves MV, Burbank DE, Roth R, Heuser J, DeAngelis PL, Van Etten JL (1999) Hyaluronic acid synthesis in virus PBCV-1-infected Chlorella-like green algae. Virology 257:15–23
Heath A, DiRita VJ, Barg NL, Engleberg NC (1999) A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun 67:5298–5305
Holo H, Nes IF (1989) High-frequency transformation, by electroporation of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 55:3119–3123
Izawa N, Serata M, Sone T, Omasa T, Ohtake H (2011) Hyaluronic acid production by recombinant Streptococcus thermophilus. J Biosci Bioeng 111:665–670
Itano N, Sawai T, Yoshida M, Lena P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274:25085–25092
Jagannath S, Ramachandran KB (2010) Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Biochem Eng J 48:148–158
Kleerebezem M, van Kranenburg R, Tuinier R, Boels IC, Zoon P, Looijesteijn E, Hugenholtz J, de Vos WM (1999) Exopolysaccharides produced by Lactococcus lactis: from genetic engineering to improved rheological properties? Antonie Van Leeuwenhoek 76:357–365
Kogan G, Šolté L, Stern R, Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29:17–25
Krahulec J, Tlustá M, Stuchlík S, Turňa J (2011) Structure of the has operon promoter and the effect of mutations on the has promoter strength in Streptococcus equi subsp. zooepidemicus. Mol Biotechnol 49:166–175
Kuipers OP, de Ruyter P, Kleerebezem M, de Vos WM (1998) Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21
Levin JC, Wessels MR (1998) Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol 30:209–219
Mao Z, Chen R (2007) Recombinant synthesis of hyaluronan by Agrobacterium sp. Biotechnol Prog 23:1038–1042
Mao Z, Shin HD, Chen R (2009) A recombinant E. coli bioprocess for hyaluronan synthesis. Appl Microbiol Biotechnol 84:63–69
Marcellin E, Gruber CW, Archer C, Craik DJ, Nielsen LK (2009a) Proteome analysis of the hyaluronic acid-producing bacterium, Streptococcus zooepidemicus. Proteome Sci 7:13
Marcellin E, Nielsen LK, Abeydeera P, Krömer JO (2009b) Quantitative analysis of intracellular sugar phosphates and sugar nucleotides in encapsulated streptococci using HPAEC-PAD. Biotechnol J 4:58–63
Necas J, Bartosikova L, Brauner P, Kolar J (2008) Hyaluronic acid (hyaluronan): a review. Vet Med 53:397–411
Prasad SB, Jayaraman G, Ramachandran KB (2010) Hyaluronic acid production is enhanced by the additional co-expression of UDP-glucose pyrophosphorylase in Lactococcus lactis. Appl Microbiol Biotechnol 86:273–283
Prasad S. B. (2012) Metabolic engineering of Lactococcus lactis for hyaluronan production. PhD thesis, Department of Biotechnology, IIT Madras, India
Ramos A, Boels IC, de Vos WM, Santos H (2001) Relationship between glycolysis and exopolysacharide biosynthesis in Lactococcus lactis. Appl Environ Microbiol 67:33–41
Rangaswamy V, Jain D (2008) An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol Lett 30:493–496
Sanghe, N., S. B. Prasad, K. B. Ramachandran and G. Jayaraman (2011) Hyaluronic acid production in metabolically engineered Lactococcus lactis NZ9000: bioreactor studies and flux balance analysis, 1st European Congress of Applied Biotechnology, Berlin, Germany
Sheng JZ, Ling PX, Zhu XQ, Guo XP, Zhang TM, He YL, Wang FS (2009) Use of induction promoters to regulate hyaluronan synthesis and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lactis: a case study of the regulation mechanism of hyaluronic acid polymer. J Appl Microbiol 107:136–144
Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, Tammi MI (2011) Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J 278:1419–1428
Tu CX, Zhang RX, Zhang XJ, Huang T (2009) Exogenous N-acetylglucosamine increases hyaluronan production in cultured human dermal fibroblasts. Arch Dermatol 301:549–551
van Kranenburg R, Boels IC, Kleerebezem M, de Vos WM (1999) Genetics and engineering of microbial exopolysaccharides for food: approaches for the production of existing and novel polysaccharides. Curr Opin Biotechnol 10:498–504
Volpi N, Schiller J, Stern R, Soltes L (2009) Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem 16:1718–1745
Widner B, Behr R, Dollen SV, Tang M, Heu T, Sloma A, Sternberg D, De Angelis PL, Weigel PH, Brown S (2005) Hyaluronic acid production in Bacillus subtilis. Appl Environ Microbiol 77:3747–3752
Wu TF, Huang WC, Chen YC, Tsay YG, Chang CS (2009) Proteomic investigation of the impact of oxygen on the protein profiles of hyaluronic acid-producing Streptococcus zooepidemicus. Proteomics 9:4507–4518
Yu H, Stephanopoulos G (2008) Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab Eng 10:24–32
Acknowledgements
The authors would like to acknowledge the financial support provided by Department of Science and Technology, Government of India, under WOS-A scheme for this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Comparative genomic analysis of three strains of S. zooepidemicus and L. lactis MG1363 for the presence of genes of the HA biosynthetic pathway (MW molecular mass, pI isoelectric point, CDS coding sequence, bp basepair, AA amino acid residue) (DOC 96 kb)
Rights and permissions
About this article
Cite this article
Prasad, S.B., Ramachandran, K.B. & Jayaraman, G. Transcription analysis of hyaluronan biosynthesis genes in Streptococcus zooepidemicus and metabolically engineered Lactococcus lactis . Appl Microbiol Biotechnol 94, 1593–1607 (2012). https://doi.org/10.1007/s00253-012-3944-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3944-0