Summary
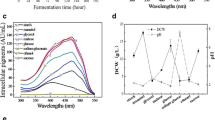
New water-soluble red pigments were produced byMonascus sp. in a chemically defined fermentation medium containing glutamate as nitrogen source. They were isolated and characterized as glutamate derivatives of the well-known orangeMonascus pigments (monascorubrin and rubropunctatin). The new pigments have several advantages over the known redMonascus pigments (rubropunctamine and monascorubramine) including very high water-solubility, higher absorption coefficient, and greater resistance to decoloration by light. Adding glutamate, glycine or leucine to a resting-cell system led to the formation of specific water-soluble red pigments corresponding to the exogenous amino acid. The water-soluble red pigments produced by resting-cells have retention times identical to those of the corresponding red derivatives made chemically from the orange pigments in methanol-phosphate buffer at pH 7. The hydrophobicities of the amino acid sources correspond to the HPLC retention times of the red pigments derived from them.
Similar content being viewed by others
References
Bender, M.L., R.J. Bergeron and M. Komiyama. 1984. General acid-base catalysis: organic reactions. In: The Bioorganic Chemistry of Enzymatic Reactions. pp 71–115. John Wiley & Sons, New York.
Büchi, H., J.D. White and G.N. Wogan. 1965. The structures of mitorubrin and mitorubrinol. J. Am. Chem. Soc. 87: 3484–3489.
Carey, F.A. and R.J. Sundberg. 1984. Advanced Organic Chemistry. Part A: Structure and Mechanism. pp. 403–454. Plenum Press, New York.
Chang, R.Q. 1977. Studies on the stability ofMonascus pigment. Masters Thesis. National Taiwan University, Taipei, Taiwan (in Chinese).
Cordes, E.H. and W.P. Jencks. 1962. On the mechanism of Schiff base formation and hydrolysis. J. Am. Chem. Soc. 84: 832–837.
Endo, N. and S. Koyama. 1977. Water soluble pigment ofMonascus. Japan Patent Kokai 77,034,986.
Francis, F.J. 1987. Lesser-known food colorants, Food Technol. 41: 62–68.
Haws, E.J., J.S.E. Holker, A. Kelly, A.D.G. Powell and A. Robertson. 1959. The chemistry of fungi. Part 37. The structure of rubropunctatin. J. Chem. Soc. 3598–3610.
Huang, T.L. 1981. Fermentative production and toxic test of natural pigment-Monascus pigments. Masters Thesis, National Taiwan University, Taipei, Taiwan (in Chinese).
Kumasaki, S., K. Nakanishi, E. Nishikawa and M. Ohashi. 1962. Structure of monascorubrin. Tetrahedron 18: 1171–1184.
Lin, T.F. and C.T. Huang. 1983. The zymotic properties ofMonascus mold. The production of extracellular amylases. Ann. Rpt. Res. Inst. Wines 157–167 (in Chinese).
Lin, T.F. and A.L. Demain. 1991. Effect of nutrition ofMonascus sp. on formation of red pigments. Appl. Microbiol. Biotechnol. 36: 70–75.
Moll, H.R. and D.R. Farr. 1976. Red pigment and process. U.S. Patent 3,993,789.
Nakagawa, N., S. Watanabe and J. Kobayashi. 1980. Nucleotide treatment ofMonascus pigments to produce meat-coloring agents. Japan Patent Kokai 80,09,682.
Pellian, K.A. and R.E. Tellis. 1971. Dyes, methods of analysis. In: Encyclopedia of Industrial Chemical Analysis. Vol 12, (F.R. Snell and L.S. Ettre, eds.) 11–13. Interscience, New York.
Ritchie, C.D. 1990. Physical Organic Chemistry. 2nd edn., 227–252. Marcel Dekker, New York.
Spears, K. 1988. Developments in food colorings: the natural alternatives. Tibtech 6: 283–288.
Su, Y.C. and W.H. Wang. 1983. Chinese red rice: Anka. In: Handbook of Indigenous Fermented Foods. (K.H. Steinkraus, R.E. Cullen, C.S. Pederson, L.F. Nellis and B.K. Gavitt, eds.), 547–553. Marcel Dekker, New York.
Sweeny, J.G., M.C. Estrada-Valdes, G.A. lacobucci, H. Sato and S. Sakamura. 1981. Photoprotection of the red pigments ofMonascus anka in aqueous media by 1,4,6-trihydroxynaphthalene. J. Agric. Food Chem. 29: 1189–1193.
Tezuka, T. and M. Kashino. 1979. Heat-stable food-coloring agent. Japan Patent Kokai 79,086,669.
Toyo Jozo Co., Ltd. 1976a,b. Water-solubleMonascus pigment. Japan Patent Kokai 76,091,937 and 76,091,938.
Toyo Jozo Co., Ltd. 1978. Food coloring. Japan Patent 5,306,003.
Toyo Jozo Co., Ltd. 1980.Monascus pigment production. Japan Patent Kokai 80,102,660.
Whalley, W.B. 1963. The sclerotiorin group of fungal metabolites: their structure and biosynthesis. Pure & Appl. Chem. 7: 565–587.
Wong, H.C. 1982. Antibiotic and pigment production byMonascus purpureus. Ph. D. Thesis, University of Georgia, Athens.
Yamaguchi, Y., H. Ito, S. Watanabe, T. Yoshida and A. Komatsu. 1973. Water-solubleMonascus pigment. U.S. Patent 3,765,906.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, T.F., Yakushijin, K., Büchi, G.H. et al. Formation of water-solubleMonascus red pigments by biological and semi-synthetic processes. Journal of Industrial Microbiology 9, 173–179 (1992). https://doi.org/10.1007/BF01569621
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569621