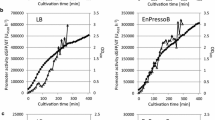
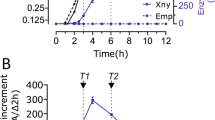
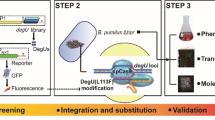
Abstract
The first whole transcriptome assessment of a Bacillus megaterium strain provides unanticipated insights into the degSU regulon considered to be of central importance for exo-enzyme production. Regulatory patterns as well as the transcription of degSU itself deviate from the model organism Bacillus subtilis; the number of DegU-regulated secretory enzymes is rather small. Targets for productivity optimization, besides degSU itself, arise from the unexpected DegU-dependent induction of the transition-state regulator AbrB during exponential growth. Induction of secretion-assisting factors, such as the translocase subunit SecY or the signal peptidase SipM, promote hypersecretion. B. megaterium DegSU transcriptional control is advantageous for production purposes, since the degU32 constitutively active mutant conferred hypersecretion of a heterologous Bacillus amyloliquefaciens amylase without the detrimental rise, as for B. subtilis and Bacillus licheniformis, in extracellular proteolytic activities.








Similar content being viewed by others
References
Amati G, Bisicchia P, Galizzi A (2004) DegU-P represses expression of the motility fla-che operon in Bacillus subtilis. J Bacteriol 186(18):6003–6014
Ashiuchi M, Nakamura H, Yamamoto M, Misono H (2006) Novel poly-γ-glutamate-processing enzyme catalyzing γ -glutamyl dd-amidohydrolysis. J Biosci Bioeng 102(1):60–65
Banse AV, Chastanet A, Rahn-Lee L, Hobbs EC, Losick R (2008) Parallel pathways of repression and antirepression governing the transition to stationary phase in Bacillus subtilis. Proc Natl Acad Sci U S A 105(40):15547–15552
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340(4):783–795
Biedendieck R (2006) Bacillus megaterium: versatile tools for production, secretion and purification of recombinant proteins. Doctoral thesis, Department of Life Sciences, TU Braunschweig
Biedendieck R, Yang Y, Deckwer WD, Malten M, Jahn D (2007) Plasmid system for the intracellular production and purification of affinity-tagged proteins in Bacillus megaterium. Biotechnol Bioeng 96(3):525–537
Biedendieck R, Borgmeier C, Bunk B, Stammen S, Scherling C, Meinhardt F, Wittmann C, Jahn D (2011) Systems biology of recombinant protein production using Bacillus megaterium. Methods Enzymol in press
Borgmeier C, Voigt B, Hecker M, Meinhardt F (2011) Functional analysis of the response regulator DegU in Bacillus megaterium DSM319 and comparative secretome analysis of degSU mutants. Appl Microbiol Biotechnol 91(3):699–711
Brown BJ, Carlton BC (1980) Plasmid-mediated transformation in Bacillus megaterium. J Bacteriol 142(2):508–512
Bunk B (2010) Comparative and functional genomics of Bacillus megaterium DSM319. Doctoral thesis, Department of Biology, TU Braunschweig
Dahl MK, Msadek T, Kunst F, Rapoport G (1991) Mutational analysis of the Bacillus subtilis DegU regulator and its phosphorylation by the DegS protein kinase. J Bacteriol 173(8):2539–2547
Dahl MK, Msadek T, Kunst F, Rapoport G (1992) The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J Biol Chem 267(20):14509–14514
Eppinger M, Bunk B, Johns MA, Edirisinghe JN, Kutumbaka KK, Koenig SSK, Creasy HH, Rosovitz MJ, Riley DR, Daugherty S, Martin M, Elbourne LDH, Paulsen I, Biedendieck R, Braun C, Grayburn S, Dhingra S, Lukyanchuk V, Ball B, Ul-Qamar R, Seibel J, Bremer E, Jahn D, Ravel J, Vary PS (2011) Genome sequences of the biotechnologically important B. megaterium strains QM B1551 and DSM319. J Bacteriol 193(16):4199–4213
Frees D, Savijoki K, Varmanen P, Ingmer H (2007) Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol Microbiol 63(5):1285–1295
Fujita M, Gonzalez-Pastor JE, Losick R (2005) High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol 187(4):1357–1368
Gärtner D, Geissendorfer M, Hillen W (1988) Expression of the Bacillus subtilis xyl operon is repressed at the level of transcription and is induced by xylose. J Bacteriol 170(7):3102–3109
Gerth U, Kirstein J, Mostertz J, Waldminghaus T, Miethke M, Kock H, Hecker M (2004) Fine-tuning in regulation of Clp protein content in Bacillus subtilis. J Bacteriol 186(1):179–191
Hamoen LW, Van Werkhoven AF, Venema G, Dubnau D (2000) The pleiotropic response regulator DegU functions as a priming protein in competence development in Bacillus subtilis. Proc Natl Acad Sci U S A 97(16):9246–9251
Härtig E, Geng H, Hartmann A, Hubacek A, Münch R, Ye RW, Jahn D, Nakano MM (2004) Bacillus subtilis ResD induces expression of the potential regulatory genes yclJK upon oxygen limitation. J Bacteriol 186(19):6477–6484
Hoffmann K (2010) Promotorstudien zur Analyse des Zwei-Komponenten-Regulationssystems DegS/DegU in Bacillus megaterium. Master thesis, Department of Biology, WWU Münster
Hoffmann K, Wollherr A, Larsen M, Rachinger M, Liesegang H, Ehrenreich A, Meinhardt F (2010) Facilitation of direct conditional knockout of essential genes in Bacillus licheniformis DSM13 by comparative genetic analysis and manipulation of genetic competence. Appl Environ Microbiol 76(15):5046–5057
Homann A, Biedendieck R, Götze S, Jahn D, Seibel J (2007) Insights into polymer versus oligosaccharide synthesis: mutagenesis and mechanistic studies of a novel levansucrase from Bacillus megaterium. Biochem J 407(2):189–198
Hunger W, Claus D (1981) Taxonomic studies on Bacillus megaterium and on agarolytic Bacillus strains. In: Goodfellow RCWBM (ed) The aerobic endospore- forming bacteria: classification and identification. Academic Press, London, pp 217–239
Hyyrylainen HL, Sarvas M, Kontinen VP (2005) Transcriptome analysis of the secretion stress response of Bacillus subtilis. Appl Microbiol Biotechnol 67(3):389–396
Jers C, Kobir A, Sondergaard EO, Jensen PR, Mijakovic I (2011) Bacillus subtilis two-component system sensory kinase DegS is regulated by serine phosphorylation in its input domain. PLoS One 6(2):e14653
Jürgen B, Hanschke R, Sarvas M, Hecker M, Schweder T (2001) Proteome and transcriptome based analysis of Bacillus subtilis cells overproducing an insoluble heterologous protein. Appl Microbiol Biotechnol 55(3):326–332
Kawamura F, Doi RH (1984) Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol 160(1):442–444
Kobayashi K (2007) Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol Microbiol 66(2):395–409
Lammers M, Nahrstedt H, Meinhardt F (2004) The Bacillus megaterium comE locus encodes a functional DNA uptake protein. J Basic Microbiol 44(6):451–458
Lee JS, Wittchen KD, Stahl C, Strey J, Meinhardt F (2001) Cloning, expression, and carbon catabolite repression of the bamM gene encoding β-amylase of Bacillus megaterium DSM319. Appl Microbiol Biotechnol 56(1–2):205–211
Mäder U, Antelmann H, Buder T, Dahl MK, Hecker M, Homuth G (2002) Bacillus subtilis functional genomics: genome-wide analysis of the DegS–DegU regulon by transcriptomics and proteomics. Mol Genet Genomics 268(4):455–467
Malten M, Nahrstedt H, Meinhardt F, Jahn D (2005) Coexpression of the type I signal peptidase gene sipM increases recombinant protein production and export in Bacillus megaterium MS941. Biotechnol Bioeng 91(5):616–621
Malten M, Biedendieck R, Gamer M, Drews AC, Stammen S, Buchholz K, Dijkhuizen L, Jahn D (2006) A Bacillus megaterium plasmid system for the production, export, and one-step purification of affinity-tagged heterologous levansucrase from growth medium. Appl Environ Microbiol 72(2):1677–1679
McLoon AL, Guttenplan SB, Kearns DB, Kolter R, Losick R (2011) Tracing the domestication of a biofilm-forming bacterium. J Bacteriol 193(8):2027–2034
Meinhardt F, Stahl U, Ebeling W (1989) Highly efficient expression of homologous and heterologous genes in Bacillus megaterium. Appl Microbiol Biotechnol 30(4):343–350
Meinhardt F, Busskamp M, Wittchen KD (1994) Cloning and sequencing of the leuC and nprM genes and a putative spoIV gene from Bacillus megaterium DSM319. Appl Microbiol Biotechnol 41(3):344–351
Msadek T (1999) When the going gets tough: survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol 7(5):201–207
Msadek T, Kunst F, Henner D, Klier A, Rapoport G, Dedonder R (1990) Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol 172(2):824–834
Mukai K, Kawata-Mukai M, Tanaka T (1992) Stabilization of phosphorylated Bacillus subtilis DegU by DegR. J Bacteriol 174(24):7954–7962
Murray EJ, Kiley TB, Stanley-Wall NR (2009) A pivotal role for the response regulator DegU in controlling multicellular behaviour. Microbiology 155(1):1–8
Nahrstedt H, Meinhardt F (2004) Structural and functional characterization of the Bacillus megaterium uvrBA locus and generation of UV-sensitive mutants. Appl Microbiol Biotechnol 65(2):193–199
Nahrstedt H, Wittchen K, Rachman MA, Meinhardt F (2004) Identification and functional characterization of a type I signal peptidase gene of Bacillus megaterium DSM319. Appl Microbiol Biotechnol 64(2):243–249
Nahrstedt H, Schröder C, Meinhardt F (2005a) Evidence for two recA genes mediating DNA repair in Bacillus megaterium. Microbiology SGM 151(3):775–787
Nahrstedt H, Waldeck J, Gröne M, Eichstädt R, Feesche J, Meinhardt F (2005b) Strain development in Bacillus licheniformis: construction of biologically contained mutants deficient in sporulation and DNA repair. J Biotechnol 119(3):245–254
Ogura M, Tsukahara K (2010) Autoregulation of the Bacillus subtilis response regulator gene degU is coupled with the proteolysis of DegU-P by ClpCP. Mol Microbiol 75(5):1244–1259
Ogura M, Yamaguchi H, Yoshida K, Fujita Y, Tanaka T (2001) DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B.subtilis two-component regulatory systems. Nucleic Acids Res 29(18):3804–3813
Ogura M, Shimane K, Asai K, Ogasawara N, Tanaka T (2003) Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol Microbiol 49(6):1685–1697
Phillips ZE, Strauch MA (2002) Bacillus subtilis sporulation and stationary phase gene expression. Cell Mol Life Sci 59(3):392–402
Pohl S, Harwood CR (2010) Heterologous protein secretion by Bacillus species from the cradle to the grave. Adv Appl Microbiol 73:1–25
Richhardt J (2009) Transkonjugation als genetisches Werkzeug für Bacillus megaterium: Optimierung und direkter Knock-out von Genen. Master thesis, Department of Biology, WWU Münster
Richhardt J, Larsen M, Meinhardt F (2010) An improved transconjugation protocol for Bacillus megaterium facilitating a direct genetic knockout. Appl Microbiol Biotechnol 86(6):1959–1965
Rygus T, Hillen W (1991) Inducible high-level expression of heterologous genes in Bacillus megaterium using the regulatory elements of the xylose-utilization operon. Appl Microbiol Biotechnol 35(5):594–599
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50(1):1–17
Schmidt S, Wolf N, Strey J, Nahrstedt H, Meinhardt F, Waldeck J (2005) Test systems to study transcriptional regulation and promoter activity in Bacillus megaterium. Appl Microbiol Biotechnol 68(5):647–655
Schumann W (2007) Production of recombinant proteins in Bacillus subtilis. Adv Appl Microbiol 62:137–189
Shimane K, Ogura M (2004) Mutational analysis of the helix-turn-helix region of Bacillus subtilis response regulator DegU, and identification of cis-acting sequences for DegU in the aprE and comK promoters. J Biochem 136(3):387–397
Shimizu K, Nakamura H, Ashiuchi M (2007) Salt-inducible bionylon polymer from Bacillus megaterium. Appl Environ Microbiol 73(7):2378–2379
Simonen M, Palva I (1993) Protein secretion in Bacillus species. Microbiol Rev 57(1):109–137
Stammen S, Müller BK, Korneli C, Biedendieck R, Gamer M, Franco-Lara E, Jahn D (2010) High-yield intra- and extracellular protein production using Bacillus megaterium. Appl Environ Microbiol 76(12):4037–4046
Steinmetz M, Le Coq D, Aymerich S (1989) Induction of saccharolytic enzymes by sucrose in Bacillus subtilis: evidence for two partially interchangeable regulatory pathways. J Bacteriol 171(3):1519–1523
Strauch MA, Hoch JA (1993) Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol Microbiol 7(3):337–342
Strauch M, Webb V, Spiegelman G, Hoch JA (1990) The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci U S A 87(5):1801–1805
Takesue N, Sone T, Tanaka M, Tomita F, Asano K (2009) Effect of an additionally introduced degQ gene on di-D-fructofuranosyl 2,6′:2′,6 anhydride (DFA IV) production by recombinant Bacillus subtilis in a single culture production system. J Biosci Bioeng 107(6):623–629
Tobisch S, Glaser P, Krüger S, Hecker M (1997) Identification and characterization of a new β-glucoside utilization system in Bacillus subtilis. J Bacteriol 179(2):496–506
Tsukahara K, Ogura M (2008) Promoter selectivity of the Bacillus subtilis response regulator DegU, a positive regulator of the fla/che operon and sacB. BMC Microbiol 8:8
Vary PS (1994) Prime time for Bacillus megaterium. Microbiology 140(5):1001–1013
Vary PS, Biedendieck R, Fuerch T, Meinhardt F, Rohde M, Deckwer WD, Jahn D (2007) Bacillus megaterium—from simple soil bacterium to industrial protein production host. Appl Microbiol Biotechnol 76(5):957–967
Veening JW, Igoshin OA, Eijlander RT, Nijland R, Hamoen LW, Kuipers OP (2008) Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol Syst Biol 4:184
Verhamme DT, Kiley TB, Stanley-Wall NR (2007) DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol Microbiol 65(2):554–568
Vorob’eva IP, Khmel IA, Alföldi L (1980) Polyethylene glycol induction of Bacillus megaterium protoplast transformation by plasmid DNA. Dokl Akad Nauk SSSR 251(4):977–980
Waldeck J, Meyer-Rammes H, Wieland S, Feesche J, Maurer KH, Meinhardt F (2007) Targeted deletion of genes encoding extracellular enzymes in Bacillus licheniformis and the impact on the secretion capability. J Biotechnol 130(2):124–132
Wang W, Sun J, Hollmann R, Zeng AP, Deckwer WD (2006) Proteomic characterization of transient expression and secretion of a stress-related metalloprotease in high cell density culture of Bacillus megaterium. J Biotechnol 126(3):313–324
Westers H, Darmon E, Zanen G, Veening JW, Kuipers OP, Bron S, Quax WJ, van Dijl JM (2004) The Bacillus secretion stress response is an indicator for α-amylase production levels. Lett Appl Microbiol 39(1):65–73
Westers H, Westers L, Darmon E, van Dijl JM, Quax WJ, Zanen G (2006) The CssRS two-component regulatory system controls a general secretion stress response in Bacillus subtilis. Febs J 273(16):3816–3827
Wittchen KD, Meinhardt F (1995) Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl Microbiol Biotechnol 42(6):871–877
Wittchen KD, Strey J, Bültmann A (1998) Molecular characterization of the operon comprising the spoIV gene of Bacillus megaterium DSM319 and generation of a deletion mutant. J Gen Appl Microbiol 44(5):317–326
Woodcock DM, Crowther PJ, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith SS, Michael MZ, Graham MW (1989) Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res 17(9):3469–3478
Wu XC, Lee W, Tran L, Wong SL (1991) Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J Bacteriol 173(16):4952–4958
Yang YH, Paquet AC (2005) Preprocessing two-color spotted arrays. In: Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S (eds) Bioinformatics and computational biology solutions using R and bioconductor, 1st edn. Springer, New York, pp 49–69
Yansura DG, Henner DJ (1984) Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A 81(2):439–443
Yasumura A, Abe S, Tanaka T (2008) Involvement of nitrogen regulation in Bacillus subtilis degU expression. J Bacteriol 190(15):5162–5171
Acknowledgements
This work was supported by the Federal Ministry of Education and Research (BMBF), grant no. 0315283. This work was financially supported by “Deutsche Forschungsgemeinschaft (SFB578)”.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00253-011-3655-y
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(JPEG 67 kb)
High resolution image
(TIFF 20137 kb)
Supplementary Tables
(DOC 478 kb)
Rights and permissions
About this article
Cite this article
Borgmeier, C., Biedendieck, R., Hoffmann, K. et al. Transcriptome profiling of degU expression reveals unexpected regulatory patterns in Bacillus megaterium and discloses new targets for optimizing expression. Appl Microbiol Biotechnol 92, 583–596 (2011). https://doi.org/10.1007/s00253-011-3575-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3575-x