Abstract
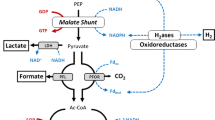
When attempting to increase yields of desirable end-products during fermentation, there is the possibility that increased concentrations of one product redirects metabolism towards the synthesis of less desired products. Changes in growth, final end-product concentrations, and activities of enzymes involved in pyruvate catabolism and fermentative end-product formation were studied in Clostridium thermocellum in response to the addition of individual end-products (H2, acetate, ethanol, formate, and lactate) to the growth medium. These were added to the growth medium at concentrations ten times greater than those found at the end of growth in cultures grown under carbon-limited conditions using cellobiose (1.1 g l−1) as model soluble substrate. Although growth rate and final cell biomass decreased significantly with the addition of all end-products, addition of individual end-products had less pronounced effects on growth. Metabolic shifts, represented by changes in final end-product concentrations, were observed; H2 and acetate yields increased in the presence of exogenous ethanol and lactate, while ethanol yields increased in the presence of exogenous hydrogen (H2), acetate, and lactate. Late exponential phase enzyme activity data of enzymes involved in pyruvate catabolism and end-product formation revealed no changes in enzyme levels greater than 2-fold in response to the presence of any given end-product, with the exception of pyruvate:formate lyase (PFL), ferredoxin-dependent hydrogenase (Fd-H2ase), and pyruvate:ferredoxin oxidoreductase (PFO): PFL and Fd-H2ase activities increased 2-fold in the presence of ethanol, while PFO activity decreased by 57% in the presence of sodium formate. Changes in enzyme levels did not necessarily correlate with changes in final end-product yields, suggesting that changes in final end-product yields may be governed by thermodynamic considerations rather than levels of enzyme expressed under the conditions tested. We demonstrate that bacterial metabolism may be manipulated in order to selectively improve desired product yields.


Similar content being viewed by others
References
Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Domiguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22:477–485
Ben-Bassat A, Lamed R, Zeikus JG (1981) Ethanol production by thermophilic bacteria: metabolic control of end product formation in Thermoanaerobium brockii. J Bacteriol 146:192–199
Bothun GD, Knutson BL, Berberich JA, Strobel HJ, Nokes SE (2004) Metabolic selectivity and growth of Clostridium thermocellum in continuous culture under elevated hydrostatic pressure. Appl Microbiol Biotechnol 65:149–157
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254
Carere CR, Kalia V, Sparling R, Cicek N, Levin DB (2008) Pyruvate catabolism and hydrogen synthesis pathway genes of Clostridium thermocellum ATCC 27405. Indian J Microbiol 48:252–266
Daniels L, Wessels D (1984) A method for the spectrophotometric assay of anaerobic enzymes. Anal Biochem 141:232–237
Daniels L, Rajagopal BS, Belay N (1986) Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol 51:703–709
Darrett RH, Grisham CM (1995) Biochemistry. Saunders College, New York
Demain AL, Newcomb M, Wu JH (2005) Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev 69:124–154
Desvaux M, Guedon E, Petitdemange H (2001) Metabolic flux in cellulose batch and cellulose-fed continuous cultures of Clostridium cellulolyticum in response to acidic environment. Microbiology 147:1461–1471
DOE-JGI (2008) http://genome.jgi-psf.org/cloth/cloth.annotation.html
Freier D, Mothershed CP, Wiegel J (1988) Characterization of Clostridium thermocellum JW20. Appl Environ Microbiol 54:204–211
Guedon E, Payot S, Desvaux M, Petitdemange H (1999) Carbon and electron flow in Clostridium cellulolyticum grown in chemostat culture on synthetic medium. J Bacteriol 181:3262–3269
Hallenbeck PC (2005) Fundamentals of the fermentative production of hydrogen. Water Sci Technol 52:21–29
He Q, Lokken PM, Chen S, Zhou J (2009) Characterization of the impact of acetate and lactate on ethanolic fermentation by Thermoanaerobacter ethanolicus. Bioresour Technol 100:5955–5965
Herrero AA, Gomez RF, Roberts MF (1985a) 31P NMR studies of Clostridium thermocellum. Mechanism of end product inhibition by ethanol. J Biol Chem 260:7442–7451
Herrero AA, Gomez RF, Roberts MF (1985b) Growth inhibition of Clostridium thermocellum by carboxylic acids: a mechanism based on uncoupleing by weak acids. Appl Microbiol Biotechnol 22:53–62
Islam R, Cicek N, Sparling R, Levin D (2006) Effect of substrate loading on hydrogen production during anaerobic fermentation by Clostridium thermocellum 27405. Appl Microbiol Biotechnol 72:576–583
Kell DB, Peck MW, Rodger G, Morris JG (1981) On the permeability to weak acids and bases of the cytoplasmic membrane of Clostridium pasteurianum. Biochem Biophys Res Commun 99:81–88
Kurose N, Yagyu J, Ohmori M, Matsumoto M, Ohsato K, Yamada T, Uchida M, Obayashi A (1989) Characterization of new strains of Clostridium thermocellum and the celA gene from a strain. Agric Biol Chem 53:3179–3185
Lamed R, Zeikus JG (1980) Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J Bacteriol 144:569–578
Lamed RJ, Lobos JH, Su TM (1988) Effects of stirring and hydrogen on fermentation products of Clostridium thermocellum. Appl Environ Microbiol 54:1216–1221
Lin WR, Peng Y, Lew S, Lee CC, Hsu JJ, Hamel J-F, Demain AL (1988) Purification and characterization of acetate kinase from Clostridium thermocellum. Tetrahedron 54:15915–15925
Lovitt RW, Shen GJ, Zeikus JG (1988) Ethanol production by thermophilic bacteria: biochemical basis for ethanol and hydrogen tolerance in Clostridium thermohydrosulfuricum. J Bacteriol 170:2809–2815
Lynd LR, Grethlein HE (1987) Hydrolysis of dilute acid pretreated mixed hardwood and purified microcrystalline cellulose by cell-free broth from Clostridium thermocellum. Biotechnol Bioeng 29:92–100
Lynd LR, Grethlein HE, Wolkin RH (1989) Fermentation of cellulosic substrates in batch and continuous culture by Clostridium thermocellum. Appl Environ Microbiol 55:3131–3139
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577, table of contents
Ozkan M, Yilmaz EI, Lynd LR, Ozcengiz G (2004) Cloning and expression of the Clostridium thermocellum L-lactate dehydrogenase gene in Escherichia coli and enzyme characterization. Can J Microbiol 50:845–851
Pei J, Zhou Q, Jiang Y, Le Y, Li H, Shao W, Wiegel J (2010) Thermoanaerobacter spp. control ethanol pathway via transcriptional regulation and versatility of key enzymes. Metab Eng 12:420–428
Pei J, Zhou Q, Jing Q, Li L, Dai C, Li H, Wiegel J, Shao W (2011) The mechanism for regulating ethanol fermentation by redox levels in Thermoanaerobacter ethanolicus. Metab Eng 13:186–193
Rydzak T, Levin DB, Cicek N, Sparling R (2009) Growth phase-dependent enzyme profile of pyruvate catabolism and end-product formation in Clostridium thermocellum ATCC 27405. J Biotechnol 140:169–175
Sanders R (1999) Compilation of Henry’s law constants for inorganic and organic species of potential importance in environmental chemistry. Air Chemistry Department, Max-Planck Institute of Chemistry. Mainz, Germany
Soboh B, Linder D, Hedderich R (2004) A multisubunit membrane-bound [NiFe] hydrogenase and an NADH-dependent Fe-only hydrogenase in the fermenting bacterium Thermoanaerobacter tengcongensis. Microbiology 150:2451–2463
Sparling R, Islam R, Cicek N, Carere C, Chow H, Levin DB (2006) Formate synthesis by Clostridium thermocellum during anaerobic fermentation. Can J Microbiol 52:681–688
Stevenson DM, Weimer PJ (2005) Expression of 17 genes in Clostridium thermocellum ATCC 27405 during fermentation of cellulose or cellobiose in continuous culture. Appl Environ Microbiol 71:4672–4678
Strobel HJ (1995) Growth of the thermophilic bacterium Clostridium thermocellum on continuous culture. Curr Microbiol 31:210–214
Strobel HJ, Caldwell FC, Dawson KA (1995) Carbohydrate transport by the anaerobic thermophile Clostridium thermocellum LQRI. Appl Environ Microbiol 61:4012–4015
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Weimer PJ, Zeikus JG (1977) Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol 33:289–297
Zheng X-J, Yu H-Q (2005) Inhibitory effects of butyrate on biological hydrogen production with mixed anaerobic cultures. J Environ Manage 74:65–70
Zhang Y-HP, Lynd LR (2005) Cellulose utilization by Clostridium thermocellum: bioenergetics and hydrolysis product assimilation. PNAS 102:7321–7325
Acknowledgements
This work was supported by funds provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), through a Strategic Programs grant (STPGP 306944–04) and the BIOCAP Canada Foundation, as well as Genome Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rydzak, T., Levin, D.B., Cicek, N. et al. End-product induced metabolic shifts in Clostridium thermocellum ATCC 27405. Appl Microbiol Biotechnol 92, 199–209 (2011). https://doi.org/10.1007/s00253-011-3511-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3511-0