Abstract
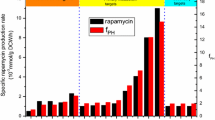
Rapamycin is a macrocyclic polyketide with immunosuppressive, antifungal, and anticancer activity produced by Streptomyces hygroscopicus ATCC 29253. Rapamycin production by a mutant strain (UV2-2) induced by ultraviolet mutagenesis was improved by approximately 3.2-fold (23.6 mg/l) compared to that of the wild-type strain. The comparative analyses of gene expression and intracellular acyl-CoA pools between wild-type and the UV2-2 strains revealed that the increased production of rapamycin in UV2-2 was due to the prolonged expression of rapamycin biosynthetic genes, but a depletion of intracellular methylmalonyl-CoA limited the rapamycin biosynthesis of the UV2-2 strain. Therefore, three different metabolic pathways involved in the biosynthesis of methylmalonyl-CoA were evaluated to identify the effective precursor supply pathway that can support the high production of rapamycin: propionyl-CoA carboxylase (PCC), methylmalonyl-CoA mutase, and methylmalonyl-CoA ligase. Among them, only the PCC pathway along with supplementation of propionate was found to be effective for an increase in intracellular pool of methylmalonyl-CoA and rapamycin titers in UV2-2 strain (42.8 mg/l), indicating that the PCC pathway is a major methylmalonyl-CoA supply pathway in the rapamycin producer. These results demonstrated that the combined approach involving traditional mutagenesis and metabolic engineering could be successfully applied to the diagnosis of yield-limiting factors and the enhanced production of industrially and clinically important polyketide compounds.






Similar content being viewed by others
References
Aparicio JF, Molnár I, Schwecke T, König A, Haydock SF, Khaw LE, Staunton J, Leadlay PF (1996) Organization of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of the enzymatic domains in the modular polyketide synthase. Gene 169:9–16
Arndt C, Cruz MC, Carnenas ME, Meitman J (1999) Secretion of FK506/FK520 and rapamycin by Streptomyces inhibits the growth of competing Saccharomyces cerevisiae and Cryptococcus neoformans. Microbiology 145:1989–2000
Baltz RH (2001) Genetic methods and strategies for secondary metabolite yield improvement in actinomycetes. Antonie Leeuwenhoek 79:251–259
Calne RY, Collier DS, Lim S, Pollard SG, Samaan A, White DJG, Thiru S (1989) Rapamycin for immunosuppression in organ allografting. Lancet 120:443–444
Chen X, Wei P, Fan L, Yang D, Zhu X, Shen W, Xu Z, Cen P (2009) Generation of high-yield rapamycin-producing strains through protoplasts-related techniques. Appl Microbiol Biotechnol 83:507–512
Cheng YR, Fang A, Demain AL (1995) Effect of amino acids on rapamycin biosynthesis by Streptomyces hygroscopicus. Appl Microbiol Biotechnol 43:1096–1098
Cheng YR, Juang J, Qiang H, Lin WL, Demain AL (2001) Mutagenesis of the rapamycin producer Streptomyces hygroscopicus FC904. J Antibiot 11:967–972
Douros J, Suffness M (1981) New antitumor substances of natural origin. Cancer Treat Rev 8:63–69
Gregory MA, Gaisser S, Lill RE, Hong H, Sheridan RM, Wilkinson B, Petkovic H, Weston AJ, Carletti I, Lee HL, Staunton J, Leadlay PF (2004) Isolation and characterization of pre-rapamycin, the first macrocyclic intermediate in the biosynthesis of the immunosuppressant rapamycin by S. hygroscopicus. Angew Chem Int Ed 43:2551–2553
Gregory MA, Hong H, Lill RE, Gaisser S, Petkovic H, Low L, Sheehan LS, Carletti I, Ready SJ, Ward MJ, Kaja AL, Weston AJ, Challis IR, Leadlay PF, Martin CJ, Wilkinson B, Sheridan RM (2006) Rapamycin biosynthesis: elucidation of gene product function. Org Biomol Chem 4:3565–3568
Horswill RA, Escalante-Semerena JC (1999) The prpE gene of Salmonella typhimurium LT2 encodes propionyl-CoA synthetase. Microbiology 145:1381–1388
Jung WS, Jeong SJ, Park SR, Choi CY, Park BC, Park JW, Yoon YJ (2008) Enhanced heterologous production of desosaminyl macrolides and their hydroxylated derivatives by overexpression of the pikD regulatory gene in Streptomyces venezuelae. Appl Environ Microbiol 74:1972–1979
Khaw LE, Böhm GA, Metcalfe S, Staunton J, Leadlay PF (1998) Mutational biosynthesis of novel rapamycins by a strain of Streptomyces hygroscopicus NRRL 5491 disrupted in rapL, encoding a putative lysine cyclodeaminase. J Bacteriol 180:809–814
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hoopwood DA (2000) Practical Streptomyces genetics. John Innes Centre, Norwich
Kojima I, Cheng YR, Mohan V, Demain AL (1995) Carbon source nutrition of rapamycin biosynthesis in Streptomyces hygroscopicus. J Ind Microbiol 14:436–439
König A, Schwecke T, Molnár I, Böhm GA, Lowden PAS, Staunton J, Leadlay PF (1997) The pipecolate-incorporating enzyme for the biosynthesis of the immunosuppressant rapamycin. Eur J Biochem 247:526–534
Kuščer E, Coates N, Challis I, Gregory M, Wilkinson B, Sheridan R, Petković H (2007) Roles of rapH and rapG in positive regulation of rapamycin biosynthesis in Streptomyces hygroscopicus. J Bacteriol 189:4756–4763
Lee MS, Kojima I, Demain AL (1997) Effect of nitrogen source on biosynthesis of rapamycin by Streptomyces hygroscopicus. J Ind Microbiol Biotechnol 19:83–86
Lum AM, Huang J, Hutchinson R, Kao CM (2004) Reverse engineering of industrial pharmaceutical-producing antinomycete strains using DNA microarrays. Metab Eng 6:186–196
Mo S, Ban YH, Park JW, Yoo YJ, Yoon YJ (2009) Enhanced FK506 production in Streptomyces clavuligerus CKD1119 by engineering the supply of methylmalonyl-CoA precursor. J Ind Microbiol Biotechnol 36:1473–1482
Molnár I, Aparicio JF, Haydock SF, Khaw LE, Schwecke T, König A, Staunton J, Leadlay PF (1996) Organization of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of genes flanking the polyketide synthase. Gene 169:1–7
Murli S, Kennedy J, Dayem LC, Carney JR, Kealey JT (2003) Metabolic engineering of Escherichia coli for improved 6-deoxyerythronolide B production. J Ind Microbiol Biotechnol 30:500–509
Olano C, Lombó F, Méndez C, Salas JA (2008) Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng 10:281–292
Park JW, Jung WS, Park SR, Park BC, Yoon YJ (2007) Analysis of intracellular short organic acid-coenzyme A esters from actinomycetes using liquid chromatography-electrospray ionization-mass spectrometry. J Mass Spectrom 42:1136–1147
Park SR, Han AR, Ban YH, Yoo YJ, Kim EJ, Yoon YJ (2010) Genetic engineering of macrolide biosynthesis: past advances, current state, and future prospects. Appl Microbiol Biotechnol 85:1227–1239
Reeves AR, Cernota WH, Brikun IA, Wesley RK, Weber JM (2004) Engineering precursor flow for increased erythromycin production in Aeromicrobium erythreum. Metab Eng 6:300–312
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2006) Effects of methylmalonyl-CoA mutase gene knockouts on erythromycin production in carbohydrate-based and oil-based fermentations of Saccharopolyspora erythraea. J Ind Microbiol Biotechnol 33:600–609
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2007) Engineering of the methylmalonyl-CoA metabolite node of Saccharopolyspora erythraea for increased erythromycin production. Metab Eng 9:293–303
Rodriguez E, Gramajo H (1999) Genetic and biochemical characterization of the α and β components of a propionyl-CoA carboxylase complex of Streptomyces coelicolor A3(2). Microbiology 145:3109–3119
Rokem JS, Lantz AE, Nielsen J (2007) Systems biology of antibiotic production by microorganisms. Nat Prod Rep 24:1262–1287
Schewecke T, Aparicio JF, Molnár I, König A, Khaw LE, Haydock SF, Oliynyk M, Caffrey P, Cortés J, Lester JB, Böhm GA, Staunton J, Leadlay PF (1995) The biosynthetic gene cluster for the polyketide immunosuppressant rapamycin. Proc Natl Acad Sci USA 92:7839–7843
Sehgal SN, Baker H, Vezina C (1975) Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot 28:727–733
Vrijbloed JW, Zerbe-Burkhardt K, Ratnatilleke A, Grubelnik-Leiser A, Robinson JA (1999) Insertional inactivation of methylmalonyl coenzyme A (CoA) mutase and isobutyryl-CoA mutase genes in Streptomyces cinnamonensis: influence on polyketide antibiotic biosynthesis. J Bacteriol 181:5600–5605
Xu ZN, Shen WH, Chen XY, Lin JP, Cen PL (2005) A high-throughput method for screening of rapamycin-producing strains of Streptomyces hygroscopicus by cultivation in 96-well microtiter plates. Biotechnol Lett 27:1135–1140
Zhu X, Zhang W, Chen X, Wu H, Duan Y, Xu Z (2010) Generation of high rapamycin producing strain via rational metabolic pathway-based mutagenesis and further titer improvement with fed-batch bioprocess optimization. Biotechnol Bioeng 107:506–515
Acknowledgments
This work was supported by the National Research Laboratory (NRL) program (R0A-2008-000-20030-0) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST), the Advanced Biomass R&D Center (ABC) funded by MEST (ABC-2010-0029800), and CKD Bio Advanced Technology Center funded by the Ministry of Knowledge Economy, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Won Seok Jung and Young Ji Yoo contributed equally to the work.
Rights and permissions
About this article
Cite this article
Jung, W.S., Yoo, Y.J., Park, J.W. et al. A combined approach of classical mutagenesis and rational metabolic engineering improves rapamycin biosynthesis and provides insights into methylmalonyl-CoA precursor supply pathway in Streptomyces hygroscopicus ATCC 29253. Appl Microbiol Biotechnol 91, 1389–1397 (2011). https://doi.org/10.1007/s00253-011-3348-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3348-6