Abstract
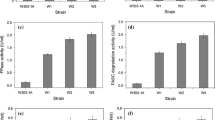
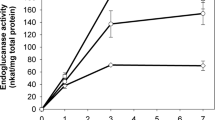
We demonstrate the value of the thermotolerant yeast Issatchenkia orientalis as a candidate microorganism for bioethanol production from lignocellulosic biomass with the goal of consolidated bioprocessing. The I. orientalis MF-121 strain is acid tolerant, ethanol tolerant, and thermotolerant, and is thus a multistress-tolerant yeast. To express heterologous proteins in I. orientalis, we constructed a transformation system for the MF-121 strain and then isolated the promoters of TDH1 and PGK1, two genes that were found to be strongly expressed during ethanol fermentation. As a result, expression of β-glucosidase from Aspergillus aculeatus could be achieved with I. orientalis, demonstrating successful heterologous gene expression in I. orientalis for the first time. The transformant could convert cellobiose to ethanol under acidic conditions and at high temperature. Simultaneous saccharification and fermentation (SSF) was performed with the transformant, which produced 29 g l−1 of ethanol in 72 h at 40°C even without addition of β-glucosidase when SSF was carried out in medium containing 100 g l−1 of microcrystalline cellulose and a commercial cellulase preparation. These results suggest that using a genetically engineered thermotolerant yeast such as I. orientalis in SSF could lead to cost reduction because less saccharification enzymes are required.




Similar content being viewed by others
References
Ballesteros I, Ballesteros M, Cabañas A, Carrasco J, Martín C, Negro MJ, Saez F, Saez R (1991) Selection of thermotolerant yeasts for simultaneous saccharification and fermentation (SSF) of cellulose to ethanol. Appl Biochem Biotechnol 28–29:307–315
Ballesteros I, Oliva JM, Ballesteros M, Carrasco J (1993) Optimization of the simultaneous saccharification and fermentation process using thermotolerant yeasts. Appl Biochem Biotechnol 39–40:201–211
Banat IM, Nigam P, Marchant R (1992) Isolation of thermotolerant, fermentative yeasts growing at 52°C and producing ethanol at 45°C and 50°C. World J Microbiol Biotechnol 8:259–283
Boeke JD, LaCroute F, Fink GR (1984) A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet 197:345–346
Bollók M, Réczey K, Zacchi G (2000) Simultaneous saccharification and fermentation of steam-pretreated spruce to ethanol. Appl Biochem Biotechnol 84–86:69–80
Chen X, Fang H, Rao Z, Shen W, Zhuge B, Wang Z, Zhuge J (2008) An efficient genetic transformation method for glycerol producer Candida glycerinogenes. Microbiol Res 163:531–537
Daniel HM, Vrancken G, Takrama JF, Camu N, De Vos P, De Vuyst L (2009) Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res 9:774–783
De Backer MD, Maes D, Vandoninck S, Logghe M, Contreras R, Luyten WH (1999) Transformation of Candida albicans by electroporation. Yeast 15:1609–1618
Dmytruk OV, Dmytruk KV, Abbas CA, Voronovsky AY, Sibirny AA (2008) Engineering of xylose reductase and overexpression of xylitol dehydrogenase and xylulokinase improves xylose alcoholic fermentation in the thermotolerant yeast Hansenula polymorpha. Microb Cell Fact 7:21
Faber KN, Haima P, Harder W, Veenhuis M, AB G (1994) Highly-efficient electrotransformation of the yeast Hansenula polymorpha. Curr Genet 25:305–310
Fujita Y, Takahashi S, Ueda M, Tanaka A, Okada H, Morikawa Y, Kawaguchi T, Arai M, Fukuda H, Kondo A (2002) Direct and efficient production of ethanol from cellulosic material with a yeast strain displaying cellulolytic enzymes. Appl Environ Microbiol 68:5136–5141
Fujita Y, Ito J, Ueda M, Fukuda H, Kondo A (2004) Synergistic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of an engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl Environ Microbiol 70:1207–1212
Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS (2003) Global analysis of protein expression in yeast. Nature 425:737–741
Gietz RD, Schiestl RH (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2:31–34
Haas LO, Cregg JM, Gleeson MA (1990) Development of an integrative DNA transformation system for the yeast Candida tropicalis. J Bacteriol 172:4571–4577
Hisamatsu M, Furubayashi T, Karita S, Mishima T, Isono N (2006) Isolation and identification of a novel yeast fermenting ethanol under acidic conditions. J Appl Glycosci 53:111–113
Hisatomi T, Kuroyanagi S, Tsuboi M (1998) Identification of the orotidine-5′-phosphate decarboxylase gene and development of a transformation system in the yeast Saccharomyces exiguus Yp74L-3. Biosci Biotechnol Biochem 62:2280–2282
Hong J, Wang Y, Kumagai H, Tamaki H (2007) Construction of thermotolerant yeast expressing thermostable cellulase genes. J Biotechnol 130:114–123
Katemai W, Maneerat S, Kawai F, Kanzaki H, Nitoda T, H-Kittikun A (2008) Purification and characterization of a biosurfactant produced by Issatchenkia orientalis SR4. J Gen Appl Microbiol 54:79–82
Kawaguchi T, Enoki T, Tsurumaki S, Sumitani J, Ueda M, Ooi T, Arai M (1996) Cloning and sequencing of the cDNA encoding β-glucosidase 1 from Aspergillus aculeatus. Gene 173:287–288
Koh JH, Suh HJ (2009) Biological activities of thermo-tolerant microbes from fermented rice bran as an alternative microbial feed additive. Appl Biochem Biotechnol 157:420–430
Li Y, Shen W, Wang Z, Liu JQ, Rao Z, Tang X, Fang H, Zhuge J (2005) Isolation and sequence analysis of the gene URA3 encoding the orotidine-5′-phosphate decarboxylase from Candida glycerinogenes WL2002-5, an industrial glycerol producer. Yeast 22:423–430
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotechnol Bioeng Symp 6:21–33
Nonklang S, Abdel-Banat BM, Cha-aim K, Moonjai N, Hoshida H, Limtong S, Yamada M, Akada R (2008) High-temperature ethanol fermentation and transformation with linear DNA in the thermotolerant yeast Kluyveromyces marxianus DMKU3-1042. Appl Environ Microbiol 74:7514–7521
Nonklang S, Ano A, Abdel-Banat BM, Saito Y, Hoshida H, Akada R (2009) Construction of flocculent Kluyveromyces marxianus strains suitable for high-temperature ethanol fermentation. Biosci Biotechnol Biochem 73:1090–1095
Prillinger H, Molnár O, Eliskases-Lechner F, Lopandic K (1999) Phenotypic and genotypic identification of yeasts from cheese. Antonie Van Leeuwenhoek 75:267–283
Rajoka MI, Shaukat F, Ghauri MT, Shahid R (2003) Kinetics of β-glucosidase production by Saccharomyces cerevisiae recombinants harboring heterologous bgl genes. Biotechnol Lett 25:945–948
Rajoka MI, Ferhan M, Khalid AM (2005) Kinetics and thermodynamics of ethanol production by a thermotolerant mutant of Saccharomyces cerevisiae in a microprocessor-controlled bioreactor. Lett Appl Microbiol 40:316–321
Sakai Y, Kazarimoto T, Tani Y (1991) Transformation system for an asporogenous methylotrophic yeast, Candida boidinii: cloning of the orotidine-5′-phosphate decarboxylase gene (URA3), isolation of uracil auxotrophic mutants, and use of the mutants for integrative transformation. J Bacteriol 173:7458–7463
Sánchez M, Iglesias FJ, Santamaría C, Domínguez A (1993) Transformation of Kluyveromyces lactis by electroporation. Appl Environ Microbiol 59:2087–2092
Schwarz WH, Gräbnitz F, Staudenbauer WL (1986) Properties of a Clostridium thermocellum endoglucanase produced in Escherichia coli. Appl Environ Microbiol 51:1293–1299
Seo SH, Rhee CH, Park HD (2007) Degradation of malic acid by Issatchenkia orientalis KMBL 5774, an acidophilic yeast strain isolated from Korean grape wine pomace. J Microbiol 45:521–527
Shaw AJ, Podkaminer KK, Desai SG, Bardsley JS, Rogers SR, Thorne PG, Hogsett DA, Lynd LR (2008) Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. Proc Natl Acad Sci U S A 105:13769–13774
Shimoda C, Itadani A, Sugino A, Furusawa M (2006) Isolation of thermotolerant mutants by using proofreading-deficient DNA polymerase delta as an effective mutator in Saccharomyces cerevisiae. Genes Genet Syst 81:391–397
Sridhar M, Sree NK, Rao LV (2002) Effect of UV radiation on thermotolerance, ethanol tolerance and osmotolerance of Saccharomyces cerevisiae VS1 and VS3 strains. Bioresour Technol 83:199–202
Suga M, Hatakeyama T (2001) High efficiency transformation of Schizosaccharomyces pombe pretreated with thiol compounds by electroporation. Yeast 18:1015–1021
Suryawati L, Wilkins MR, Bellmer DD, Huhnke RL, Maness NO, Banat IM (2008) Simultaneous saccharification and fermentation of Kanlow switchgrass pretreated by hydrothermolysis using Kluyveromyces marxianus IMB4. Biotechnol Bioeng 101:894–902
Tailliez P, Girard H, Millet J, Beguin P (1989) Enhanced cellulose fermentation by an asporogenous and ethanol-tolerant mutant of Clostridium thermocellum. Appl Environ Microbiol 55:207–211
Thalagala TATP, Kodama S, Mishima T, Isono N, Furujyo A, Kawasaki Y, Hisamatsu M (2009) Study on ethanol fermentation using D-glucose rich fractions obtained from lignocelluloses by a two-step extraction with sulfuric acid and Issatchenkia orientalis MF 121. J Appl Glycosci 56:7–11
Tokuhiro K, Ishida N, Kondo A, Takahashi H (2008) Lactic fermentation of cellobiose by a yeast strain displaying beta-glucosidase on the cell surface. Appl Microbiol Biotechnol 79:481–488
Tomás-Pejó E, García-Aparicio M, Negro MJ, Oliva JM, Ballesteros M (2009) Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresour Technol 100:890–895
Van Bogaert IN, De Maeseneire SL, De Schamphelaire W, Develter D, Soetaert W, Vandamme EJ (2007) Cloning, characterization and functionality of the orotidine-5'-phosphate decarboxylase gene (URA3) of the glycolipid-producing yeast Candida bombicola. Yeast 24:201–208
van Zyl WH, Lynd LR, den Haan R, McBride JE (2007) Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Adv Biochem Engin/Biotechnol 108:205–235
Wang Z, Zhuge J, Fang H (1999) A new osmotolerant and glycerol-highly-producing species—Candida glycerolgenesis Zhuge sp. nov. Wei Sheng Wu Xue Bao 39:68–74
Wang X, Li G, Deng Y, Yu X, Chen F (2005) A site-directed integration system for the nonuniversal CUG(Ser) codon usage species Pichia farinosa by electroporation. Arch Microbiol 184:419–424
Watanabe I, Nakamura T, Shima J (2009) Characterization of a spontaneous flocculation mutant derived from Candida glabrata: a useful strain for bioethanol production. J Biosci Bioeng 107:379–382
Yamagata K, Kato J, Shimamoto A, Goto M, Furuichi Y, Ikeda H (1998) Bloom's and Werner's syndrome genes suppress hyperrecombination in yeast sgs1 mutant: implication for genomic instability in human diseases. Proc Natl Acad Sci U S A 95:8733–8738
Yang VW, Marks JA, Davis BP, Jeffries TW (1994) High-efficiency transformation of Pichia stipitis based on its URA3 gene and a homologous autonomous replication sequence, ARS2. Appl Environ Microbiol 60:4245–4254
Zhou J, Dong Z, Liu L, Du G, Chen J (2009) A reusable method for construction of non-marker large fragment deletion yeast auxotroph strains: a practice in Torulopsis glabrata. J Microbiol Methods 76:70–74
Zhuge J, Fang HY, Wang ZX, Chen DZ, Jin HR, Gu HL (2001) Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl Microbiol Biotechnol 55:686–692
Acknowledgments
The pYO323 plasmid vector (NBRP ID number BYP563) of YEp type harboring the HIS3 marker was kindly donated by the National Bioresource Project (Yeast) of Japan (http://yeast.lab.nig.ac.jp/nig/index_en.html). We also thank Risa Nagura, Satoshi Katahira, and Takashi Matsuyama for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Takao Kitagawa and Kenro Tokuhiro contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kitagawa, T., Tokuhiro, K., Sugiyama, H. et al. Construction of a β-glucosidase expression system using the multistress-tolerant yeast Issatchenkia orientalis . Appl Microbiol Biotechnol 87, 1841–1853 (2010). https://doi.org/10.1007/s00253-010-2629-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2629-9