Abstract
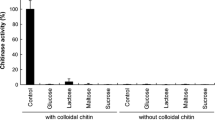
The mesophilic organism, Oerskovia xanthineolytica NCIM 2839, was adapted to grow at moderate thermophilic temperatures. At these elevated temperatures, it was found to produce two thermostable chitinases—C1 and C2. These were purified by ion exchange chromatography using DEAE cellulose. The chitinases C1 and C2 were found to be stable in a pH range from 3.0 to 9.0 with 7.5 and 8.0 being the optimum pH, respectively. The optimum temperatures of the activities of C1 and C2 were 50 and 55°C, respectively. These were activated by Mn++ and Cu++and inactivated by Hg++. This is first report of an extracellular thermostable chitinase being produced by O. xanthineolytica NCIM 2839.











Similar content being viewed by others
References
Bhushan B (2000) Production and characterization of a thermostable chitinase from new alkalophilic Bacillus sp. BG-11. J Appl Microbiol 88:800–808
Cabib E (1987) The synthesis and degradation in chitin. Adv Enzymol 59:59–101
Flach J, Pilet PE, Jolles P (1992) What’s new in chitinase research? Experientia 48:701–716
Giambattista RD, Federici F, Petruccioli M, Fenice M (2001) The chitinolytic activity of Penicillium janthinellum P9: purification, partial characterization and potential applications. J Appl Microbiol 91:498–505
Gomes RC, Seamedo LTAS, Soares RMA, Linhares LF, Ulhoa CJ, Alviano CS, Coelho RRR (2001) Purification of a thermostable endochitinase from Streptomyces RC1071 isolated from a cerrado soil and its antagonism against phytopathogenic fungi. J Appl Microbiol 90:653–661
Gohel V, Naseby DC (2007) Thermal stabilization of chitinolytic enzymes of Pantoea dispersa. Journal Of Biochemical Engineering 35:150–157
Hiroshi T, Katsuhiko M, Katsushiro M, Hiroshi E, Masafumi M, Yoshihiko I (1993) Purification and properties of a thermostable chitinase from Streptomyces thermoviolaceus OPC-520. Appl Environ Microbiol 59:620–622
Hsu SC, Lockwood JL (1975) Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. J Appl Microbiol 29:422–426
Iverson KL, Bromel MC, Anderson AW, Freeman TP (1984) Bacterial symbionts in the sugar beet root maggots Tetanops myopaeformis. Appl Environ Microbiol 47:22–27
Kenji S, Akira Y, Hajime K, Mamoru W, Mitsuaki M (1998) Purification and characterization of three thermostable endochitinases of a noble Bacillus strain, MH-1, isolated from chitin containing—compost. Appl Environ Microbiol 64:3397–3402
Kramer KJ, Koga D (1986) Insect chitin: physical state, synthesis, degradation and metabolic regulation. Insect Biochem 16:851–877
Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lien TS, Yu ST, Wu ST, Too JR (2007) Induction and purification of thermophilic chitinase produced by Aeromonas sp. DYU 007 using glucosamine. Biotechnol Bioprocess Eng 12:610–617
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mann JW, Jeffries TW, Macmillan JD (1978) Production and ecological significance of yeast cell wall degrading enzymes from Oerskovia. Appl Environ Microbiol 36:594–605
Merril CR (1987) Detection of proteins separated by electrophoresis. Advance Electrophoresis 1:111–139
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Muzzarelli RAA (1996) Chitosan-based dietary foods. Carbohydr Polym 29:309–316
Nam-Soo K, Kim S (1991) Some molecular characteristics and improving methods for thermal stability of enzyme. Korean Journal of Applied Microbiology Biotechnology 19:100–108
Nawani NN, Kapadnis BP (2005) Optimization of chitinase production using statistics based experimental designs. Process Biochem 40:651–660
Nawani NN, Kapadnis BP, Das AD, Rao AS, Mahajan SK (2002) Purification and characterization of a thermophilic and acidophilic chitinase from Microbispora sp.V2. J Appl Microbiol 93:965–975
Ohno T, Armand S, Hata T (1996) A modular family 19 chitinase found in the prokaryotic organism Streptomyces griseus HUT 6037. J Bacteriol 178:5065–5070
Okazaki K, Kato F, Watanabe N, Yasuda S, Masui Y, Hayakawa S (1995) Purification and properties of two chitinases from Streptomyces sp. J-13–3. Biosci Biotechnol Biochem 59:1586–1587
Purwani EY, Maggy TS, Yaya R, Jae KH, Yu RP (2004) Characteristics of thermostable chitinase enzymes from the Indonesian Bacillus sp. 13.26. Enzyme Microb Technol 35:147–153
Sakai K, Narihara M, Kasama Y, Wakayama M, Moriguchi M (1994) Purification and characterization of thermostable β-N-Acetylglucosaminidase of Bacillus stearothermophilus CH-4 isolated from chitin containing compost. Appl Environ Microbiol 60:2911–2915
Sakai K, Yokota A, Kurukowa H, Wakayama M, Moriguchi M (1998) Purification and characterization of three thermostable endochitinases of a noble Bacillus strain, MH-1, isolated from chitin containing compost. Appl Environ Microbiol 64:3397–3402
Suresh PV, Chandrasekaran M (1999) Impact of process parameters on chitinase production by an alkalophlic Beauveria bassiana in solid state fermentation. Process Biochem 34:257–267l
Takayanagi T, Ajisaka K, Takiguchi Y, Shimahara K (1991) Isolation and characterization of thermostable chitinases from Bacillus licheniformis X-7u. Biochim Biophys Acta 1078:404–410
Ueno H, Miyashita K, Sawada Y, Oba Y (1990) Purification and some properties of extracellular chitinases from Streptomyces sp. S-84. J Gen Appl Microbiol 36:377–392
Wang S, Moync A, Thottappilly G, Wu S, Locy RD, Singh NK (2001) Purification and characterization of a Bacillus cereus exochitinase. Enzyme Microb Technol 28:492–498
Wang SL, Chao CH, Liang TW, Chen CH (2008) Purification and characterization of protease and chitinase from Bacillus cereus TKU006 and conversion of marine wastes by these enzymes. Mar Biotechnol 11:334–344
Acknowledgement
This work was supported by the Department of Microbiology, Shivaji University, Kolhapur and Department of Biochemistry, Shivaji University, Kolhapur, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waghmare, S.R., Ghosh, J.S. Study of thermostable chitinases from Oerskovia xanthineolytica NCIM 2839. Appl Microbiol Biotechnol 86, 1849–1856 (2010). https://doi.org/10.1007/s00253-009-2432-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2432-7