Abstract
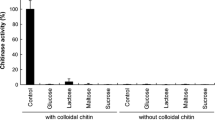
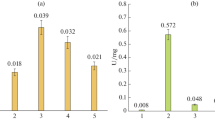
Thermostable exochitinase was purified from a culture medium of a moderately thermophilic strain Cohnella sp. IB-P192 via ultrafiltration, affinity sorption, and hydrophobic chromatography and was then characterized. Enzyme synthesis was induced by colloidal chitin from carb shells. It reached the highest level at 50°C in 72 h of submerged cultivation. The molecular weight of the purified chitinase as determined with SDS-PAGE was 69 kDa. The enzyme had pH and temperature optima of 7.5 and 70°C, respectively. It retained 100% activity under 65°C and was stable at a pH of 5–10.5. The Michaelis–Menten constant and specific Vmax of the purified chitinase were 0.83 mg × mL–1 and 116.75 μM-eqv × mL–1 × min–1 × mg–1, respectively. The enzyme was inhibited by Ag+ and Hg+2 cations and insignificantly inhibited by 1 mM Cu+2 and Ni+2, while 1 mM Mn+2, Ca+2 and Co+2 cations and Tween-80 increased its activity. The chitinase hydrolyzed specific substrate according to the exomechanism of substrate hydrolysis, forming (GlcNAc)2 as main reaction product and it functioned as N-acetyl-β-D-glucosaminidase at a later stage of hydrolysis (3–4 h). The highest rate of chitosan hydrolysis by the enzyme was recorded at a deacetylation degree (DD) of 50% at 70°C and an [E]/[S] ration of 1 : 60. The fungicidal effect of produced chitosan oligomers depended on the DD of the original polymer and most strongly increased under the destruction of the chitosan with a DD of 50%.









Similar content being viewed by others
REFERENCES
Haki, G.D. and Rakshit, S.K., Bioresour. Technol., 2003, vol. 89, no. 1, pp. 17–34. https://doi.org/10.1016/s0960-8524(03)00033-6
Vieille, C. and Zeikus, G.J., Microbiol. Mol. Biol. Rev., 2001, vol. 65, no. 1, pp. 1–43. https://doi.org/10.1128/MMBR.65.1.1-43.2001
Zeldes, B.M., Keller, M.W., Loder, A.J., Straub, C.T., Adams, M.W.W., and Kelly, R.M., Front. Microbiol., 2015, vol. 6, p. 1209. https://doi.org/10.3389/fmicb.2015.01209
Mathew, G.M., Madhavan, A., Arun, K.B., Sindhu, R., Binod, P., Singhania, R.R., Sukumaran, R.K., and Pandey, A., Appl. Biochem. Biotechnol., 2021, vol. 193, no. 1, pp. 142–164. https://doi.org/10.1007/s12010-020-03416-5
Kumar, M., Vivekanand, V., and Pareek, N., Environmental Microbiology and Biotechnology, Singh, A., Srivastava, S., Rathore, D., and Pant, D., Eds., Singapore: Springer, 2020. https://doi.org/10.1007/978-981-15-6021-7_7
Sakai, K., Yokota, A., Kurokawa, H., Wakayama, M., and Moriguchi, M., Appl. Environ. Microbiol., 1998, vol. 64, no. 9, pp. 3397–402. https://doi.org/10.1128/AEM.64.9.3397-3402.1998
Liang, T.-W., Chen, Y.-J., Yen, Y.-H., and Wang, S.-L., Process. Biochem., 2007, vol. 42, no. 4, pp. 527–534. https://doi.org/10.1016/j.procbio.2006.10.005
Kumar, A., Kumar, D., George, N., Sharma, P., and Gupta, N., Int. J. Biol. Macromol., 2018, vol. 109, pp. 263–272. https://doi.org/10.1016/j.ijbiomac.2017.12.024
Liaqat, F. and Eltem, R., Carbohydr. Res., 2018, vol. 184, pp. 243–259. https://doi.org/10.1016/j.carbpol.2017.12.067
Hobel, C.F., Hreggvidsson, G.O., Marteinsson, V.T., Bahrani-Mougeot, F., Einarsson, J.M., and Kristjansson, J.K., Extremophiles, 2005, vol. 9, no. 1, pp. 53–64. https://doi.org/10.1007/s00792-004-0422-3
Krolicka, M., Hinz, S.W.A., Koetsier, M.J., Joosten, R., Eggink, G., Broek, L.A.M., and Boeriu, C.G., J. Agric. Food Chem., 2018, vol. 66, no. 7 P, pp. 1658–1669. https://doi.org/10.1021/acs.jafc.7b04032
Xu, P., Ni, Z.-F., Zong, M.-H., Ou, X.-Y., Yang, J.-G., and Lou, W.-Y., Int. J. Biol. Macromol., 2020, vol. 150, pp. 9–15. https://doi.org/10.1016/j.ijbiomac.2020.02.033
Takayanagi, T., Ajisaka, K., Takiguchi, Y., and Shimahara, K., Biochim. Biophys. Acta, 1991, vol. 1078, no. 3, pp. 404–410. https://doi.org/10.1016/0167-4838(91)90163-t
Toharisman, A., Suhartono, M.T., Spindler-Barth, M., Hwang, J.-K., and Pyun, Y.-R., J. Microbiol. Biotechnol., 2005, vol. 21, no. 5, pp. 733–738. https://doi.org/10.1007/s11274-004-4797-1
Asmani, K.L., Bouacem, K., Ouelhadj, A., Yahiaoui, M., Bechami, S., Mechri, S., Jabeur, F., Taleb-Ait Menguellet, K., and Jaouadi, B., Carbohydr. Res., 2020, vol. 495, art. 108089. https://doi.org/10.1016/j.carres.2020.108089
Kämpfer, P., Rosselló-Mora, R., Falsen, E., Busse, H.J., and Tindall, B.J., Int. J. Syst. Evol. Microbiol., 2006, vol. 56, pt. 4, pp. 781–786. https://doi.org/10.1099/ijs.0.63985-0
Yoon, M.H., Ten, L.N., and Im, W.T., J. Microbiol. Biotechnol., 2007, vol. 17, no. 6, pp. 913–918.
Rastogi, G., Bhalla, A., Adhikari, A., Bischoff, K.M., Hughes, S.R., Christopher, L.P., and Sani, R.K., Bioresour. Technol., 2010, vol. 101, no. 22, pp. 8798–8806. https://doi.org/10.1016/j.biortech.2010.06.001
Golaki, B.P., Aminzadeh, S., Karkhane, A.A., Yakhchali, B., Farrokh, P., Khaleghinejad, S.H., Tehrani, A.A., and Mehrpooyan, S., Protein Expr. Purif., 2015, vol. 109, pp. 120–126. https://doi.org/10.1016/j.pep.2014.10.002
Mosallatpour, S., Aminzadeh, S., Shamsara, M., and Hajihosseini, R., Sci. Rep., vol. 9, no. 1, art. 19062. https://doi.org/10.1038/s41598-019-55587-9
Saghian, R., Mokhtari, E., and Aminzadeh, S., Sci. Rep., 2021, vol. 11, no. 1, p. 4573. https://doi.org/10.1038/s41598-021-84267-w
Aliabadi, N., Aminzadeh, S., Karkhane, A.A., and Haghbeen, K., Braz. J. Microbiol., 2016, vol. 47, no. 4, pp. 931–940. https://doi.org/10.1016/j.bjm.2016.07.009
Bergey’s Manual of Systematic Bacteriology, vol. 3: The Firmicutes, Vos, P., Garrity, G., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., and Whitman, W., Eds., Springer, 2009, 2nd ed.
Manual of Methods for General Bacteriology, Gerhardt, P., Murray, R.G.E., Costilow, R.N., Eds., Washington, DC: ASM, 1981.
Wilson, K., Curr. Protoc. Mol. Biol., 2001, vol. 56, no. 1, pp. 2.4.1–2.4.5. https://doi.org/10.1002/0471142727.mb0204s56
Lane, D.J., in Nucleic Acid Techniques in Bacterial Systematic, Stackebrandt, E. and Goodfellow, M., Eds., New York: Wiley, 1991, pp. 115–175.
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K., Mol. Biol. Evol., 2018, vol. 35, no. 6, pp. 1547–1549. https://doi.org/10.1093/molbev/msy096
Safina, V.R., Melentiev, A.I., Galimzyanova, N.F., Gilvanova, E.A., Kuzmina, L.Yu., Lopatin, S.A., Varlamov, V.P., Baymiev, A.H., and Aktuganov, G.E., Appl. Biochem. Microbiol., 2021, vol. 57, no. 5, pp. 626–635. https://doi.org/10.1134/S0003683821050124
Hëlisto, P., Aktuganov, G., Galimzianova, N., Melentjev, A., and Korpela, T., J. Chromatogr. B Biomed. Sci. Appl., 2001, vol. 758, no. 2, pp. 197–205. https://doi.org/10.1016/s0378-4347(01)00181-5
Aktuganov, G.E., Galimzyanova, N.F., Teregulova, G.A., and Melent’ev, A.I., Appl. Biochem. Microbiol., 2016, vol. 52, no. 5, pp. 531–536. https://doi.org/10.1134/S0003683816050021
Cho, E.A., Lee, J.S., Lee, K.C., Jung, H.C., Pan, J.G., and Pyun, Y.R., Int. J. Syst. Evol. Microbiol., 2007, vol. 57, pt. 12, pp. 2902–2907. https://doi.org/10.1099/ijs.0.64844-0
Kudryashova, E.B., Karlyshev, A.V., Ariskina, E.V., Streshinskaya, G.M., Vinokurova, N.G., Kopitsyn, D.S., and Evtushenko, L.I., Int. J. Syst. Evol. Microbiol., 2018, vol. 68, no. 9, pp. 2912–2917. https://doi.org/10.1099/ijsem.0.002919
Yahiaoui, M., Bouacem, K., Harir, M., Asmani, K., Mechri, S., and Jaouadi, B., in Proc. MOL2NET, Basel: MDPI by MOL2NET, 2021, vol. 6. https://doi.org/10.3390/mol2net-07-09377
Fu, X., Yan, Q., Yang, S., Yang, X., Guo, Y., and Jiang, Z., Biotechnol. Biofuels, 2014, vol. 7, p. 174. https://doi.org/10.1186/s13068-014-0174-y
Tran, T.N., Doan, C.T., Nguyen, M.T., Nguyen, V.B., Vo, T.P.K., Nguyen, A.D., and Wang, S.L., Polymers (Basel), 2019, vol. 11, no. 10, p. 1600. https://doi.org/10.3390/polym11101600
Sørlie, M., Horn, S.J., Vaaje-Kolstad, G., and Eijsink, V.G.H., React. Funct. Polym., 2020, vol. 148, art. 104488. https://doi.org/10.1016/j.reactfunctpolym.2020.104488
Varlamov, V.P., Il’ina, A.V., Shagdarova, B.Ts., Lun’kov, A.P., and Mysyakina, I.S., Usp. Biol. Khim., 2020, vol. 60, pp. 317–368.
ACKNOWLEDGMENTS
The equipment of the Agidel Center for Collective Use of the Ufa Federal Research Center of the Russian Academy of Sciences was used in the research.
Funding
The study was carried out with the financial support of the Russian Foundation for Basic Research within the framework of scientific project no. 19-34-90119 as and the state assignment of the Ministry of Education and Science of Russia, no. 075-00326-19-00 on topic no. АААА-А18-118022190098-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by V. Mittova
Rights and permissions
About this article
Cite this article
Gilvanova, E.A., Aktuganov, G.E., Safina, V.R. et al. Characterization of Thermotolerant Chitinase from the Strain Cohnella sp. IB P-192 and Its Application for the Production of Bioactive Chitosan Oligomers. Appl Biochem Microbiol 58, 143–154 (2022). https://doi.org/10.1134/S0003683822020077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822020077