Abstract
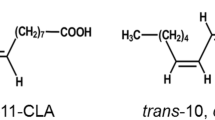
Lactobacillus plantarum AKU 1009a effectively transforms linoleic acid to conjugated linoleic acids of cis-9,trans-11-octadecadienoic acid (18:2) and trans-9,trans-11–18:2. The transformation of various polyunsaturated fatty acids by washed cells of L. plantarum AKU 1009a was investigated. Besides linoleic acid, α-linolenic acid [cis-9,cis-12,cis-15-octadecatrienoic acid (18:3)], γ-linolenic acid (cis-6,cis-9,cis-12–18:3), columbinic acid (trans-5,cis-9,cis-12–18:3), and stearidonic acid [cis-6,cis-9,cis-12,cis-15-octadecatetraenoic acid (18:4)] were found to be transformed. The fatty acids transformed by the strain had the common structure of a C18 fatty acid with the cis-9,cis-12 diene system. Three major fatty acids were produced from α-linolenic acid, which were identified as cis-9,trans-11,cis-15–18:3, trans-9,trans-11,cis-15–18:3, and trans-10,cis-15–18:2. Four major fatty acids were produced from γ-linolenic acid, which were identified as cis-6,cis-9,trans-11–18:3, cis-6,trans-9,trans-11–18:3, cis-6,trans-10–18:2, and trans-10-octadecenoic acid. The strain transformed the cis-9,cis-12 diene system of C18 fatty acids into conjugated diene systems of cis-9,trans-11 and trans-9,trans-11. These conjugated dienes were further saturated into the trans-10 monoene system by the strain. The results provide valuable information for understanding the pathway of biohydrogenation by anaerobic bacteria and for establishing microbial processes for the practical production of conjugated fatty acids, especially those produced from α-linolenic acid and γ-linolenic acid.




Similar content being viewed by others
References
Ando A, Ogawa J, Kishino S, Shimizu S (2003) CLA production from ricinoleic acid by lactic acid bacteria. J Am Oil Chem Soc 80:889–894
Ando A, Ogawa J, Kishino S, Shimizu S (2004) Conjugated linoleic acid production from castor oil by Lactobacillus plantarum JCM 1551. Enzyme Microb Technol 35:40–45
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Dawson RM, Kemp P (1969) The effect of defaunation on the phospholipids and on the hydrogenation of unsaturated fatty acids in the rumen. J Biochem 115:351–352
Destaillats F, Trottier JP, Galvez JM, Angers P (2005) Analysis of α-linolenic acid biohydrogenation intermediates in milk fat with emphasis on conjugated linolenic acids. J Dairy Sci 88:3231–3239
Griinari JM, Dwyer DA, McGuire MA, Bauman DE, Palmquist DL, Nurmela KV (1998) Trans-octadecenoic acids and milk fat depression in lactating dairy cows. J Dairy Sci 81:1251–1261
Ha YL, Grimm NK, Pariza MW (1987) Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis 8:1881–1887
Ha YL, Storkson J, Pariza MW (1990) Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res 50:1097–1101
Igarashi M, Miyazawa T (2000) Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett 148:173–179
Ip C, Chin SF, Scimeca JA, Pariza MW (1991) Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res 51:6118–6124
Jenkins TC, Wallace RJ, Moate PJ, Mosley EE (2008) Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J Anim Sci 86:397–412
Jouany JP, Lassalas B, Doreau M, Glasser F (2007) Dynamic features of the rumen metabolism of linoleic acid, linolenic acid and linseed oil measured in vitro. Lipids 42:351–360
Kepler CR, Tove SB (1967) Biohydrogenation of unsaturated fatty acids. III. Purification and properties of a linoleate Δ12-cis, Δ11-trans-isomerase from Butyrivibrio fibrisolvens. J Biol Chem 242:5686–5692
Kishino S, Ogawa J, Ando A, Omura Y, Shimizu S (2002a) Ricinoleic acid and castor oil as substrates for conjugated linoleic acid production by washed cells of Lactobacillus plantarum. Biosci Biotechnol Biochem 66:2283–2286
Kishino S, Ogawa J, Omura Y, Matsumura K, Shimizu S (2002b) Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J Am Oil Chem Soc 79:159–163
Kishino S, Ogawa J, Ando A, Iwashita T, Fujita T, Kawashima H, Shimizu S (2003) Structural analysis of conjugated linoleic acid production by Lactobacillus plantarum, and factors affecting isomer production. Biosci Biotechnol Biochem 67:179–182
Lee KN, Kritchevsky D, Pariza MW (1994) Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108:19–25
Liu L, Hammond EG, Nikolau BJ (1997) In vivo studies of the biosynthesis of α-eleostearic acid in the seed of Momordica charantia. L Plant Physiol 113:1343–1349
Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S (2001) Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl Environ Microbiol 67:1246–1252
Ostrowska E, Dunshea FR, Muralitharan M, Cross RF (2000) Comparison of silver-ion high-performance liquid chromatographic quantification of free and methylated conjugated linoleic acids. Lipids 35:1147–1153
Pariza MW, Ha YL (1990) Newly recognized anticarcinogenic fatty acids. In: Kuroda Y, Shankel D, Waters MD (eds) Antimutagenesis and anticarcinogenesis mechanism II. Plenum, New York, pp 167–170
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32:853–858
Plourde M, Destaillats F, Chouinard PY, Angers P (2007) Conjugated α-linolenic acid isomers in bovine milk and muscle. J Dairy Sci 90:5269–5275
Scollan ND, Choi NJ, Kurt E, Fisher AV, Enser M, Wood JD (2001) Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. Br J Nutr 85:115–124
Suzuki R, Noguchi R, Ota T, Abe M, Miyashita K, Kawada T (2001) Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids 36:477–482
Wasowska I, Maia MR, Niedzwiedzka KM, Czauderna M, Ribeiro JM, Devillard E, Shingfield KJ, Wallace RJ (2006) Influence of fish oil on ruminal biohydrogenation of C18 unsaturated fatty acids. Br J Nutr 95:1199–1211
Acknowledgment
This work was partially supported by the Industrial Technology Research Grant Program in 2007 (no. 07A08005a to S.K.) and the Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers (to S.S.) from New Energy and Industrial Technology Development Organization (NEDO) of Japan, Grants-in-Aid for Scientific Research (no. 19780056 to S.K., no. 16688004 to J.O., and no. 18208009 to S.S.) and COE for Microbial-Process Development Pioneering Future Production Systems from the Ministry of Education, Culture, Sports, Science and Technology, Japan. S.K. was a recipient of a Research Fellowship (no. 01985) from the Japan Society for the Promotion of Science for Yong Scientists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
1H-NMR analysis of A1, and the structure of A1 identified (GIF 345 kb)
Fig. S1
1H-NMR analysis of A1, and the structure of A1 identified (GIF 345 kb)
Fig. S3
1H-NMR analysis of CGLA2, and the structure of CGLA2 identified (GIF 368 kb)
Fig. S4
1H-NMR analysis of G2, and the structure of G2 identified (GIF 433 kb)
Fig. S5
1H-NMR analysis of G1, and the structure of G1 identified (GIF 356 kb)
Rights and permissions
About this article
Cite this article
Kishino, S., Ogawa, J., Yokozeki, K. et al. Metabolic diversity in biohydrogenation of polyunsaturated fatty acids by lactic acid bacteria involving conjugated fatty acid production. Appl Microbiol Biotechnol 84, 87–97 (2009). https://doi.org/10.1007/s00253-009-1949-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1949-0