Abstract
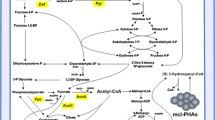
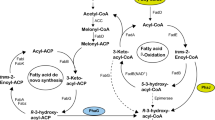
To produce extracellular chiral 3-hydroxyacyl acids (3HA) by fermentation, a novel pathway was constructed by expressing tesB gene encoding thioesterase II into Pseudomonas putida KTOY01, which was a polyhydroxyalkanoate (PHA) synthesis operon knockout mutant. 3HA mixtures of 0.35 g/l consisting of 3-hydroxyhexanoate, 3-hydroxyoctanoate, 3-hydroxydecanoate, and 3-hydroxydodecanoate (3HDD) were produced in shake-flask study using dodecanoate as a sole carbon source. Additional knockout of fadB and fadA genes encoding 3-ketoacyl-CoA thiolase and 3-hydroxyacyl-CoA dehydrogenase in P. putida KTOY01 led to the weakening of the β-oxidation pathway. The fadBA and PHA synthesis operon knockout mutant P. putida KTOY07 expressing tesB gene produced 2.44 g/l 3HA, significantly more than that of the β-oxidation intact mutant. The 3HA mixture contained 90 mol% 3HDD as a dominant component. A fed-batch fermentation process carried out in a 6-l automatic fermentor produced 7.27 g/l extracellular 3HA containing 96 mol% fraction of 3HDD after 28 h of growth. For the first time, it became possible to produce 3HDD-dominant 3HA monomers.


Similar content being viewed by others
References
Bagdasarian M, Lurz R, Ruckert B, Franklin FC, Bagdasarian MM, Frey J, Timmis KN (1981) Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237–247
Chen GQ, Wu Q (2005) Microbial production and applications of chiral hydroxyalkanoates. Appl Microbiol Biotechnol 67:592–599
De Roo G, Kellerhals MB, Ren Q, Witholt B, Kessler B (2002) Production of chiral R-3-hydroxyalkanoic acids and R-3-hydroxyalkanoic acid methyl esters via hydrolytic degradation of polyhydroxyalkanoate synthesized by Pseudomonas. Biotechnol Bioeng 77:717–722
Fiedler S, Steinbüchel A, Rehm B (2002) The role of the fatty acid β-oxidation multienzyme complex from Pseudomonas oleovorans in polyhydroxyalkanoate biosynthesis: molecular characterization of the fadBA operon from P. oleovorans and of the enoyl-CoA hydratase genes phaJ from Pseudomonas oleovorans and Pseudomonas putida. Arch Microbiol 178:149–160
Gao HJ, Wu Q, Chen GQ (2002) Enhanced production of D-(3)-3-hydroxybutyric acid by recombinant Escherichia coli. FEMS Microbiol Lett 213:59–65
Jaipuri FA, Jofre MF, Schwarz KA, Pohl NL (2004) Microwave-assisted cleavage of Weinreb amide for carboxylate protection in the synthesis of a (R)-3-hydroxyalkanoic acid. Tetrahedron Lett 45:4149–4152
Kanesawa Y, Tanahashi N, Doi Y (1994) Enzymatic degradation of microbial poly(3-hydroxyalkanoates). Polym Degrad Stab 45:179–185
Kellerhals MB, Kessler B, Witholt B (1999) Closed-loop control of bacterial high-cell-density fed-batch cultures: production of mcl-PHAs by Pseudomonas putida KT2442 under single-substrate and cofeeding conditions. Biotechnol Bioeng 65:306–315
Klinke S, Ren Q, Witholt B, Kessler B (1999) Production of medium-chain-length poly(3-hydroxyalkanoates) from gluconate by recombinant Escherichia coli. Appl Environ Microbiol 65(2):540–548
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM (1995) Four new derivatives of the broad-host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Lee SY, Lee Y (2003) Metabolic engineering of Escherichia coli for production of enantiomerically pure (R)-(−)-hydroxycarboxylic acids. Appl Environ Microbiol 69:3421–3426
Lee SY, Lee Y, Wang FL (1999) Chiral compounds from bacterial polyesters: sugars to plastics to fine chemicals. Biotechnol Bioeng 65:363–368
Lee Y, Park SH, Limb IT, Hanb K, Lee SY (2000) Preparation of alkyl (R)-(2)-3-hydroxybutyrate by acidic alcoholysis of poly-(R)-(2)-3-hydroxybutyrate. Enzyme Microb Technol 27:33–36
Liu Q, Ouyang SP, Chung A, Wu Q, Chen GQ (2007) Microbial production of R-3-hydroxybutyric acid by recombinant E. coli harboring genes of phbA, phbB, and tesB. Appl Microbiol Biotechnol 76:811–818
Magnuson K, Jackowski S, Rock CO, Cronan JE (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57:522–542
Naggert J, Narasimhan ML, DeVeaux L, Cho H, Randhawa ZI, Cronan JE, Green BN, Smith S (1991) Cloning, sequencing, and characterization of Escherichia coli thioesterase II. J Biol Chem 266:11044–11050
Ouyang SP, Liu Q, Fang L, Chen GQ (2007a) Construction of pha-operon-defined knockout mutants of Pseudomonas putida KT2442 and their applications in poly(hydroxyalkanoate) production. Macromol Biosci 7:227–233
Ouyang SP, Luo RC, Chen SS, Liu Q, Chung A, Wu Q, Chen GQ (2007b) Production of polyhydroxyalkanoates with high 3-hydroxydodecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Biomacromolecules 8:2504–2511
Park SJ, Lee SY, Lee Y (2004) Biosynthesis of (R)-3-hydroxyalkanoic acids by metabolically engineered Escherichia coli. Appl Biochem Biotechnol 113:373–379
Ren Q, Grubelnik A, Hoerler M, Ruth K, Hartmann R, Felber H, Zinn M (2005) Bacterial poly(hydroxyalkanoates) as a source of chiral hydroxyalkanoic acids. Biomacromolecules 6:2290–2298
Rehm BHA, Steinbüchel A (2001) Heterologous expression of the acyl–acyl carrier protein thioesterase gene from the plant Umbellularia californica mediates polyhydroxyalkanoate biosynthesis in recombinant Escherichia coli. Appl Microbiol Biotechnol 55(2):205–209
Ruth K, Grubelnik A, Hartmann R, Egli T, Zinn M, Ren Q (2007) Efficient production of (R)-3-hydroxycarboxylic acids by biotechnological conversion of polyhydroxyalkanoates and their purification. Biomacromolecules 8:279–286
Sandoval A, Arias-Barrau E, Bermejo F, Canedo L, Naharro G, Olivera E, Luengo J (2005) Production of 3-hydroxy-nphenylalkanoic acids by a genetically engineered strain of Pseudomonas putida. Appl Microbiol Biotechnol 67:97–105
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Seebach D, Beck AK, Breitschuh R, Job K (1992) Direct degradation of the biopolymer poly[(R)-(2)-3-hydroxybutyric acid] to (R)-(2)-3-hydroxybutanoic acid and its methyl ester. Org Synth 71:39–47
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotech 1:784–791
Spencer AK, Greenspan AD, Cronan JE (1978) Thioesterase I and II of Escherichia coli. J Biol Chem 253:5922–5926
Steinbüchel A, Füchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Takahashi K, Murakami T, Kamata A, Yumoto R, Higashi Y, Yata N (1994) Pharmacokinetic analysis of the absorption enhancing action of decanoic acid and its derivatives in rats. Pharm Res 11:388–392
Wang L, Armbruster W, Jendrossek D (2007) Production of medium-chain-length hydroxyalkanoic acids from Pseudomonas putida in pH stat. Appl Microbiol Biotechnol 75:1047–1053
Yuan MQ, Shi ZY, Wei XX, Wu Q, Chen SF, Chen GQ (2008) Microbial production of medium-chain-length3-hydroxyalkanoic acids by recombinant Pseudomonas putida KT2442 harboring genes fadL, fadD and phaZ. FEMS Microbiol Lett 283:167–175
Zhao K, Tian G, Zheng Z, Chen JC, Chen GQ (2003) Production of D-(−)-3-hydroxyalkanoic acid by recombinant Escherichia coli. FEMS Microbiol Lett 218:59–64
Zheng Z, Gong Q, Liu T, Deng Y, Chen JC, Chen GQ (2004a) Thioesterase II of Escherichia coli plays an important role in 3-hydroxydecanoic acid production. Appl Environ Microbiol 70:3807–3813
Zheng Z, Gong Q, Chen GQ (2004b) A novel method for production of 3-hydroxydecanoic acid by recombinant Escherichia coli and Pseudomonas putida. Chinese J Chem Eng 12:550–555
Zheng Z, Chen JC, Tian HL, Bei FF, Chen GQ (2005) Specific identification of (R)-3-hydroxyacyl-ACP: CoA transacylase gene from Pseudomonas and Burkholderia strains by polymerase chain reaction. Chin J Biotechnol 21:19–24
Acknowledgements
We are very grateful for the kind donation of plasmid pBBR1MCS-2 from Dr. Philip Green of Procter & Gamble (Cincinnati, OH, USA). P. putida KT2442 was a gift from Professor B. Witholt (ETH Zurich, Switzerland). This study was supported by the National High Tech 863 Grant (project no. 2006AA02Z242 and 2006AA020104), as well as the State Basic Science Foundation 973 (2007CB707804).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ahleum Chung and Qian Liu contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Chung, A., Liu, Q., Ouyang, SP. et al. Microbial production of 3-hydroxydodecanoic acid by pha operon and fadBA knockout mutant of Pseudomonas putida KT2442 harboring tesB gene. Appl Microbiol Biotechnol 83, 513–519 (2009). https://doi.org/10.1007/s00253-009-1919-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1919-6